Atoms, Molecules, Water, pH
2D says, “As caterpillars, we Monarchs love to eat the
leaves of milkweed plants. As a matter of fact, we like them so much, that’s
all we’ll eat! Don’t you go trying that, though, because there are chemicals
in milkweed leaves that are toxic to you humans. Monarchs like me can just
store those toxic chemicals in areas of our bodies where they won’t hurt us.
Actually, that works to our advantage, because if another animal tries to
eat us, we taste yucky, even as adults when we’re no longer eating the
milkweed. So, to advertize that and let everybody know how bad tasting we
are because of all those toxic chemicals we’re storing, the pigment chemicals
in our wings form bright, orange-and-black warning coloration.
As adults, like other butterflies, we drink nectar from the flowers we visit.
Of course, that nectar contains a lot of water, so just like you, we have
to have organs to deal with that water and eliminate any excess.”
Atoms and Subatomic Particles:
We need to start with a little chemistry because living
organisms are made of and use chemicals.
Individual substances are called elements, substance
which cannot be broken down or subdivided by ordinary chemical means.
We recognize about 105 or 106 elements. About 92 are natural and the rest are
man-made. These are things like oxygen, sulfur, carbon, copper, etc.
Each element has a symbol made of the first letter or two of its name. Some
are from the old Latin names: sodium = natrium, iron = ferrum, potassium =
kalium. Of the 92 naturally-occurring, four of these make up about 96% of
all living matter. These are carbon, oxygen, hydrogen, and nitrogen, and
the name COHN can help you remember these. Another 21 are needed in
smaller amounts in order to live and stay healthy.
One piece, one particle of an element is an
atom
a unit of matter or the smallest possible amount of an element. Two or more
atoms can bond together to form a
molecule.
Often the compound thus formed has properties quite different from
the elements in it. For example, sodium (Na), an extremely reactive, nearly
explosive metal, and chlorine (Cl), a toxic gas combine to form sodium
chloride (NaCl), which is common table salt.
Atoms are made up of even smaller things called
subatomic particles.
There are three main types: proton (which has a very small positive
electrical charge), neutron (which is neutral), and electron (which has a
very small, negative electrical charge). You may see these referred to as
p+, e–, and no. The protons and neutrons
form the nucleus of the atom (not to be confused with the nucleus of a cell),
while the electrons are zipping around them somewhere and are
traveling at about the speed of light.
The number of protons is important: this determines what
element something is. Each element has a different number of protons, and
if the number of protons in an atom changes, then it what element it is.
The number of protons in an atom is called its atomic number. This
is written as a subscript to the left: 8O, 6C,
etc.
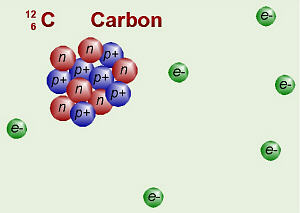
Carbon’s Subatomic Particles
Within limits, the number of neutrons and electrons in an atom can vary.
Isotopes
are atoms of the same element with different numbers of neutrons. Protons
and neutrons weigh about the same as each other, but electrons are so much
smaller, their weight is negligible by comparison (like carrying a feather
when you weigh yourself on the bathroom scale). Thus, when we want to know
how much an atom of something weighs, we can just add up the number of
protons and neutrons. The number of protons plus the number of neutrons in
an atom = its atomic weight. These are written as superscripts to the
left: 12C, 13C. Atomic weight minus atomic number
equals the number of neutrons. This means that Carbon-12 has 6 neutrons,
Carbon-13 has 7, and Carbon-14 has 8. In Carbon-14, the ratio of neutrons
to protons (8 to 6) is far enough off that the nucleus of the atom is not
stable, but undergoes radioactive decay, in which it turns into some
other chemical (beyond the scope of this course, but if you are interested,
the actual radioactive reaction, called β-emission, is
614C → 714N + e–,
and the electron that is “kicked out” is called a beta [β] particle).
The half life of a radioactive chemical is the amount of time it takes
for half of the starting quantity to undergo radioactive decay. Thus, if you
started with 1 g of a substance with a half life of 1 year, after one year,
you would have 0.5 g left, after two years you would have only 0.25 g, after
three years, 0.125 g, etc.
Molecules and Molecular Weight:
Molecular weight equals the sum of the atomic weights
of the atoms in the molecule. For NaCl, the atomic weight of sodium is 23
(in units called “Daltons”), of chlorine is 35 and a molecule contains one
sodium and one chlorine, so 23 + 35 = 58, the molecular weight of NaCl. The
formula for glucose ( a very common sugar) is
C6H12O6. The subscripts to the right mean
that it contains 6 atoms of carbon, 12 atoms of hydrogen, and 6 atoms of
oxygen. The atomic weight for carbon is 12, for hydrogen is 1, and for
oxygen is 16, so the molecular weight of glucose can be calculated thus:
| Element | Atomic
Weight | No. of
Atoms | Total
Weight |
|---|
| C | 12 | 6 | 6 | × | 12 | = 72 |
| H | 1 | 12 | 12 | × | 1 | = 12 |
| O | 16 | 6 | 6 | × | 16 | = 96 |
| Total = Molecular Weight | 180 |
|---|
Use the Periodic Table
below to help you with this
molecular weight practice problem.
(You’ll get a different problem each time you click this link.)
The numbers we will see listed on the periodic table are
averages, For example, for carbon the number given is an average atomic
weight of all the Carbon-12, Carbon-13, and Carbon-14 in the world. For our
purposes, it’s OK if you round to the nearest whole number
(C = 12, O = 16).
Electron Energy Shells:
Normally, the number of protons and electrons match so the
charge is balanced out. Sometimes, however, the number of electrons can vary.
Ions are atoms with electrons added or removed resulting in an overall
positive or negative charge.
Generally, the charge on an ion is indicated to the upper right of its symbol.
For example, a calcium ion with a +2 charge would be indicated as
Ca++ or Ca+2.
Electrons are moving rapidly all around the atom, and can possess certain
discrete quantities of energy (like making change where you might have
either a penny or a nickel or a dime, but not a 3.5 ¢ coin). These
quantities are referred to as energy shells or orbitals. These are not
orbits like the planets around the sun, but an attempt to show the energy
quantities as pictures.
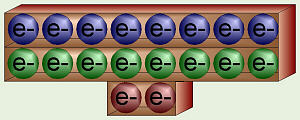
Argon’s Stable Electrons
Each energy quantity/level/shell can only have a certain number of electrons
with that much energy, kind of like if you would go to a movie where there
are 1, 5, and 10 ¢ seats. The first energy level can have two electrons
(there are two 1 ¢ seats). If there are more electrons, they must have the
next amount of energy (they have to buy 5 ¢ seats), or as a chemist would
say, they’re filling the second energy shell. Eight electrons can have the
second level of energy (eight 5 ¢ seats). If there are still more electrons
in the atoms of a particular element, then they must go into the third energy
shell (buy 10 ¢ seats) where there are another eight spots available. Beyond
that, things get too complicated for biology, so
we’ll leave that to the chemists.
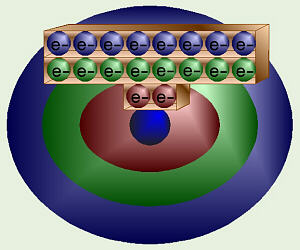
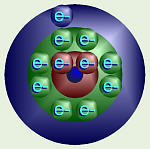
Atomic Energy Levels
Chemists, however, prefer not to think in terms of 1, 5, and 10 ¢ seats at a
movie. Often, the energy orbitals/shells are pictured as circles around the
center of the atom. Again, the electrons do NOT travel around these circles
like planets. Rather, this is just a way of showing how much energy they
have. Our movie seats would convert to “standard”; orbitals something like
this.
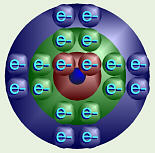
Argon’s Electron Orbitals
This is how a chemist would diagram the energy levels of this atom. Notice
that the electrons come in pairs. That idea is important in showing
how atoms bond with each other to form molecules. Thus, the first energy
level can have one pair of
electrons, the second level can have four pairs, etc.
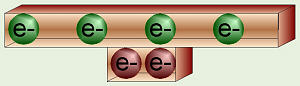
Carbon’s Balanced Electrons
As atoms of different elements gain increasing numbers of electrons, one is
put into each “pair” in a level before the second “half” of each pair is
filled. Thus, for carbon, with six protons and six electrons, two of the
electrons fill the available pair in the first energy shell. The remaining
four electrons distribute themselves, one in each of the four “pairs” in the
second level.
Electrons like to have the least amount of energy possible,
so these levels will fill “from the bottom up” (they all fill the cheap seats
first), thus not all atoms have electrons in all shells. The number of
electrons in each of the shells depends on how many total electrons they have.
This number is generally the same as the number of protons because the
electrical charge of an electron is equal but opposite to that of a proton.
Thus, hydrogen has one electron in the first level, helium has two in the
first level, lithium has two in the first and one in the second level, etc.
Note the arrangement of the periodic table: elements are organized into
columns by how many electrons they have in their outermost energy levels
(for example, H, Li, and Na each have one electron in whichever energy level
is “outermost”), and organized into rows by which energy level (1st,
2nd, etc.) they’re filling (for example, Li, C, and N all have
their first energy level full and are in various stages/numbers of electrons
of filling their second level).
Periodic Table:

Valence Electrons, Ions, and Ionic Bonds:
For any element, the electrons in the outermost energy
level/shell are the most important. These determine an element’s chemical
properties – how it will react in a chemical reaction. These important
electrons are known as the valence electrons. Know how many valence
electrons carbon, oxygen, hydrogen, nitrogen, sodium, and chlorine have.
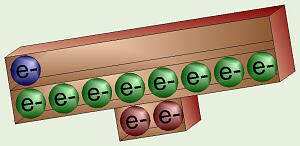
Sodium’s Unbalanced Electrons
Chlorine’s Unbalanced Electrons
All elements are most stable with a full outer energy level, whatever that
level is, so they will gain or give up electrons to make whatever is the
outermost level be full even if it doesn’t match with the number of protons
in that atom. This would be an ion, and if the atom gained electrons
to form an ion (there are more electrons than protons), then its overall
electrical charge would be negative by however many “extra” electrons it has.
If an atom gives up electrons to form an ion (there are more protons than
electrons), then its overall charge is positive.
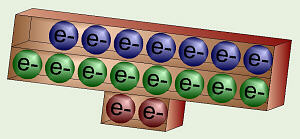
Sodium’s Electron Orbitals
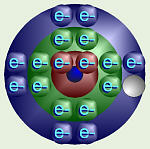
Chlorine’s Electron Orbitals
Consider sodium and chlorine. Sodium is in column I so it has one valence
electron. If it could get rid of that one, lonely electron from level 3,
then its outer level would be level 2 which is nice and full. Chlorine is
in column VII, so it has seven valence electrons, one short of a full outer
shell. Thus, it would be more stable if it could grab an electron from
somewhere to fill up that one, last spot.
Thus, when sodium and chlorine come together, in an explosive
reaction, chlorine grabs sodium’s “unwanted” electron. This forms sodium
ions with a +1 electrical charge (extra proton because it lost an electron)
and chloride ions with a –1 charge. Chemists would write this as
Na + Cl → Na+ + Cl–.
These positive and negative ions are still strongly attracted to each other,
forming ionic bonds, bonds in which one atom grabs electrons from
another. When compounds with ionic bonds are put into water, the ions come
apart and dissolve in the water.
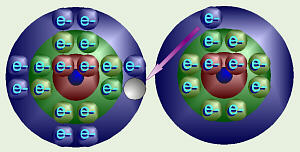
Formation of Sodium Chloride
Covalent Bonds:
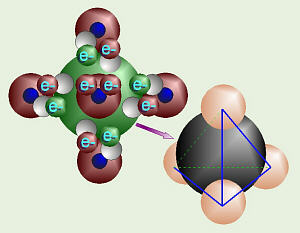
Formation of Methane
Because carbon has four valence electrons, one in each of the four “pairs,”
it is more willing to share electrons with another atom (rather than
grabbing them or giving them up). For example, methane is one atom
of carbon bonded to four atoms of hydrogen. This would have the chemical
formula, CH4. When atoms share electrons as they bond together,
this is called a covalent bond. Many compounds with covalent
(co- = with, together; valent = strength) bonds are not water
soluble. Carbon can also form covalent bonds with other atoms of carbon,
thus making long, stable chains possible. These are very important to living
organisms.
The shape of a molecule of methane is a
tetrahedron.
The hydrogen nuclei (one proton each) are all “trying” to get as close as
possible to all the electrons around the carbon, yet keep as far away as
possible from each other (like + and – poles on a magnet). In a tetrahedron,
there are four sides, all of which are triangles (in a pyramid, the bottom is
square and there are five sides). The hydrogen protons are equally spaced in
three dimensions around the carbon.
Brief Review:
As a review, we have discussed the placement of four numbers
around an element’s symbol (atomic number, atomic weight, charge, and number
of atoms in a molecule), but all four are rarely used simultaneously. Just
an an illustration, suppose a molecule of sodium carbonate is made with
radioactive sodium-24 and then dissolved in water to form sodium ions
(recall that sodium’s atomic number is 11). To indicate that there are two
atoms of that sodium in the sodium carbonate, we would write
“Na2CO3,” and if we wanted to indicate that they are sodium-24, we could rewrite this as
“24Na2CO3,” or maybe even
“1124Na2CO3.” Once the compound
is dissolved in water, forming sodium ions, we would use “Na+” or
maybe, in a rare situation, “24Na+” or
“1124Na+” to indicate those ions, but since
they are floating in the water and not attached to the carbonate
(CO3–2) ion,
now we don’t use the “2” after the “Na”. Note: if I’m using WordPerfect,
I can put the superscripts and subscripts directly over/under each other
(for example 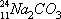 ),
but HTML doesn’t do that.
),
but HTML doesn’t do that.
Water:
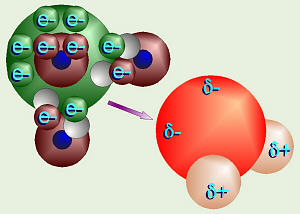
Formation of Water
The shape of a water molecule is also a tetrahedron. Oxygen has six valence
electrons and two “holes,” thus can bond with two hydrogens. Therefore, the
chemical formula for water is H2O. Oxygen’s other four valence
electrons, in two pairs, are not bonded to any other atoms, thus these are
referred to as unshared pairs of electrons. Oxygen shares electrons
with hydrogen, but pulls just a little harder on the electrons. The
electrons are just a little closer to the oxygen than the hydrogens, so this
is called a polar covalent bond. Note that even though the molecule
as a whole is electrically neutral (the + and – charges balance), the ends of
the molecule where the hydrogen nuclei are (which contain only a proton) have
a sort-of positive charge (labeled as δ+), and the ends of the molecule
by the unshared pairs of electrons are sort-of negative (labeled as δ–).
The sort-of positive ends on one water molecule are attracted to the sort-of
negative ends on another water molecule. This is called hydrogen
bonding. Actually, hydrogen bonding can happen with other molecules
besides water as we will see later.

Ice, Water, and Vapor
Water is a key ingredient in all life. Cells are 70 to 95% water. About 75%
of the Earth’s surface is covered with water. Water is the only common
substance existing naturally in all three forms: solid, liquid, and gas.
Water has many unique properties due, in great part, to its hydrogen
bonding.
- Water sticks to itself. It forms droplets. It acts like it has a film
on top.
- Water sticks to other things; well, at least some other things.
Hydrophilic
substances like glass, paper, and sugar can mix with or stick to water (yes,
on very clean glass, water “sheets” and flows over the glass without beading
up). On the other hand,
hydrophobic
substances like teflon, salad oil, and car wax will not mix with
water, and water placed on those surfaces will bead up.
- Water can absorb a lot of heat without changing temperature very much.
No, “heat” and “temperature” are not the same thing! For example, suppose
you have a Corningware® or glass pot and an aluminum pot that weigh the
same amount (contain the same amount of material). If you would place these
two pots on equal burners on the stove, which would get hot faster? Aluminum,
right? The Corningware pot can absorb more of the heat from the burner
without changing temperature as much. In the summer, in the
daytime, lakes and oceans can absorb a lot of heat from the sun, keeping the
surrounding air much cooler. At night and/or in the winter, bodies of water
will gradually give up their heat, warming the surrounding air. That’s why
costal areas tend to have more mild seasonal changes. In deserts, where
there is very little water to absorb and retain heat, summer daytime
temperatures can be extremely high, yet at night, a person would need a warm
jacket, campfire, and/or sleeping bag to keep warm in
the 40 to 50° F (5 to 10° C) weather. This property of water is also useful
in our bodies: in the summer we can absorb a lot of heat from the sun
without overheating too much, and in the winter we don’t immediately freeze
when we go outside.
- Ice floats. For most substances, the solid form is more dense than the liquid form and would sink to the bottom of any mixture of the two, but for water, the solid (ice) is less dense. If ice sank, in winter as water froze, it would sink to the
bottom of ponds and lakes, thus they would freeze from the bottom up. In spring/summer, only the top few inches would thaw because the solid ice on the bottom would never rise high enough to be warmed by the sun. Thus, it would be impossible for any
organisms to live in water.
pH:
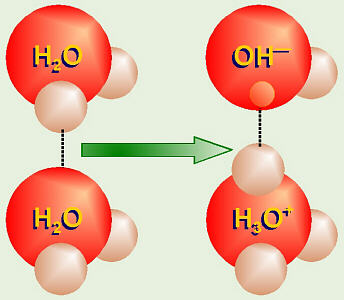
Ionization of Water: Hydronium Hydroxide
Even in plain, distilled water, because of the hydrogen bonding, sometimes
one of the hydrogen protons from one water molecule “jumps over” to one of
the pairs of unshared electrons in another water molecule (leaving its
electron behind). Thus ions of H3O+ (hydronium ion)
and OH– (hydroxide ion) are formed. This reaction would be
written as 2H2O → H3O+ + OH–.
Somebody figured out that in one liter of pure, distilled water, there will be
0.0000001 M
each of H3O+ (often written as H+) and of
OH– present.
Rather than having to write out all that, chemists came up
with the concept of
pH
as a shorthand way to keep track of how much H3O+ is
present in a solution. First, the 0.0000001 M is converted to scientific
notation, so becomes 1 × 10–7. Next the exponent or logarithm of
that number is found: the logarithm of 1 × 10–7 is simply –7.
Then, since scientists, like other people, don’t like to have to do any more
writing than necessary, the negative (–) sign is removed (a negative negative
is a positive). Thus, the –7 is changed to just a 7. Based on all of this,
pH = –log[H+],
or pH is equal to the negative logarithm of the hydrogen (hydronium) ion
concentration (“[ ]” means “the concentration of”).
If other substances are added, the concentrations of hydrogen
(hydronium) and hydroxide ions (notated as [H+] and
[OH–]) may change, but pH is always based on the hydrogen ion
concentration, [H+]. If [H+] is greater
than 0.0000001 M, (like 0.0001 or 10–4 M so pH = 4), that solution
is an acid, and if [H+] is less than 0.0000001 M, (like
0.0000000001 or 10–10 M so pH = 10) the solution is a base.
Somebody figured out that [H+] × [OH–] always equals
10–14, so if one increases, the other decreases, proportionately,
such that the product of the two will always be 10–14.
Try a pH practice problem. (You’ll get a different problem each time you click this link.)
Acids and Bases:
An acid is a substance which adds H+ to a
solution. A base is a substance which subtracts H+ from
or adds OH– to a solution. A neutral solution has a pH of 7.
If the pH of a solution is less than 7 (because [H+] is greater
than 10–7) the solution is an acid, and if the pH is greater than
7 (because [H+] is less than 10–7) the solution is a
base. Biological substances like lemon juice and vinegar are acids, and both
of these have pH values around 3. The hydrochloric acid (HCl) in toilet bowl
cleaner and in our stomachs is a strong acid — the pH of stomach acid is
between 1 and 3. Lye (NaOH), the main ingredient in many drain-openers, is
a strong base, with a pH of 12 to 14, depending on the concentration of the
solution. Bottled ammonia is also a strong base, and just its fumes are a
strong-enough base to change the color of pH indicator paper.
I have heard that the typical pH of our scalp and skin is
around 5, slightly acidic, and that pH is best for skin and scalp health and
resistance to infection and diseases. Soap and many shampoos are made via a
chemical reaction involving lye, and since there is typically a bit of
unreacted lye left in them, they are bases (some quite strong bases).
Thus, while our skin can secrete chemicals to recover its pH balance
following occasional, reasonable use of these products, too-frequent use of
these products (shampooing one’s hair on a daily basis, daily showers using
soap or detergent bars) can prevent the scalp/skin from maintaining a normal
pH, resulting in “dry” (= less healthy) skin and an increased risk of
infection. Thus, some shampoo manufacturers add chemicals to their shampoos
to lower the pH closer to the skin’s normal of pH 5, and these shampoos are
often marketed as “pH-balanced” shampoos. I have heard knowledgeable people,
including dermatologists, say that it is better for one’s skin to not
take daily showers, or at least to not use soap (just rinse with water) if
someone feels that a daily shower is a “must.” (Interestingly, while I have
not seen actual scientific studies on this, I have heard/read that our scalp
secretes chemicals which help to repel head lice, and the suggestion that
the increased incidence of head lice among school children in our country
may, in part, be related to the fact that parents are actually keeping their
children’s hair “too clean,” thereby preventing the accumulation of an
adequate supply of natural repellant on their hair.)
Buffers:
A buffer is a substance which minimizes the
change in pH or [H+]. Different buffers work best at
different pH ranges. Notice that what’s happening here is that a buffer
protects from too great of a change in pH. By no means is
this anything like the equivalent of lowering/minimizing the pH, which would
have the effect of creating a strong acid. The concept of pH and the
utilization of buffers to maintain “normal” pH ranges are important in our
bodies and in other branches of in biology. For example, an enzyme called
pepsin
digests protein in our stomachs, but must have an acid environment to
function (most of the enzymes in our bodies only function within certain pH
ranges). Not all of the food we eat is acidic, and might destroy (or
neutralize) the normal stomach pH, thus making the pepsin ineffective,
unable to digest dietary protein. To prevent this from happening, the
buffers in our stomach keep the pH fairly constant, within a range of
about pH 1 to 3. However, antacids such as Tums® or Rolaids® are so
“strong” that they overwhelm the person’s stomach’s buffers’ ability to
function properly, drastically changing the pH of the stomach contents, and
therefore, pepsin’s ability to digest the protein in one’s diet.
Calcium, by the way, is absorbed better into one’s body if the stomach
contents are acidic, thus antacids also interfere with our bodies’ ability
to properly absorb calcium. To properly absorb dietary calcium, it should
be consumed along with acidic or slightly acidic substances (such as milk
or orange juice), and not mixed in with antacids. While there may be
legitimate uses for antacids (such as when a doctor prescribes them to
assist in treating ulcers), frequent, “casual” use of antacids may actually
stimulate the production of more stomach acid as the user’s system
struggles to overcome them and return the body to normal.
As another example, our blood must remain very close to around pH 7.4, and if
it deviates too much, a person could get very sick or die. Yet, when we
transport carbon dioxide from our cells to our lungs, it turns into carbonic
acid in the deoxygenated blood. Thus, if it weren’t for buffers, our blood
would be a drastically different pH depending on how much carbon dioxide was
dissolved in it.
References:
Borror, Donald J. 1960. Dictionary of Root Words and Combining Forms. Mayfield Publ. Co.
Campbell, Neil A., Lawrence G. Mitchell, Jane B. Reece. 1999. Biology, 5th Ed. Benjamin/Cummings Publ. Co., Inc. Menlo Park, CA. (plus earlier editions)
Campbell, Neil A., Lawrence G. Mitchell, Jane B. Reece. 1999. Biology: Concepts and Connections, 3rd Ed. Benjamin/Cummings Publ. Co., Inc. Menlo Park, CA. (plus earlier editions)
Marchuk, William N. 1992. A Life Science Lexicon. Wm. C. Brown Publishers, Dubuque, IA.
Sienko, Michell J. and Robert A. Plane. 1966. Chemistry: Principles and Properties. McGraw-Hill Book Co., NY. (and other chemistry texts and handbooks)
Copyright © 1996 by J. Stein Carter. All rights reserved.
This page has been accessed  times since 15 Aug 2000.
times since 15 Aug 2000.














