Carbon-based Compounds and Functional Groups
Hydrocarbons and Alkanes:
As previously mentioned, because carbon has four valence
electrons and makes covalent bonds, it can form molecules that are long
chains. There are several different kinds of these we will be discussing:
hydrocarbons, carbohydrates,
lipids, proteins, and DNA. Because of the many
important and unique properties of carbon-based molecules, there is a
special branch of chemistry devoted just to the study of these molecules.
Organic chemistry is the study of compounds containing carbon.
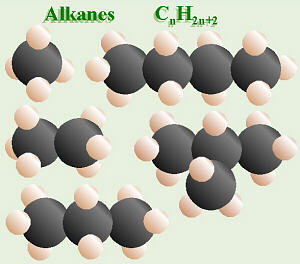
Some Alkanes
Hydrocarbons
are molecules consisting of/containing only atoms of carbon and hydrogen.
There are many different kinds of hydrocarbons based on different numbers of
carbon atoms in the molecules and whether or not any of the carbons are
connected by double bonds rather than single bonds. In double bonds,
two pairs (a total of four) electrons are shared between the two atoms
involved. Since carbon has four valence electons, it can make four single
bonds with other atoms by sharing one of its electons and one of the other
atoms’ electons with each of those atoms. In a double bond, carbon would
share two of its electrons and two electrons from another atom with that
atom (= two pairs of electrons). This would mean that this carbon atom would,
then, have only two other electrons available for bonding with any other
atoms. For example, if a carbon is single bonded to another carbon, it
could also be bonded to three hydrogens, but if a carbon is double bonded to
another carbon, then it can only bond to two hydrogens. Note that in the
word “hydrocarbon” the “hydro” part refers to hydrogen, not to water:
there is no water in hydrocarbons, only hydrogen and carbon. Generally,
hydrocarbons are not found in living organisms, but are found in quantity in
fossil fuels, which used to be living organisms. Because their structures
are the simplest to understand and because they form the building-blocks
from which other organic molecules are made, we will look first at the
structures of hydrocarbons.
Here are the names of the first ten hydrocarbons with all
single bonds. These have the general formula: CnH2n+2,
where any number could be substituted for the “n.” Note that all these
names end in “-ane,” which is an ending that signifies a hydrocarbon
with all single bonds).
| # C | Name | Wordstems |
|---|
| 1 | methane | methyl = wine |
| 2 | ethane | ether = upper air |
| 3 | propane | pro = before, in front of |
| 4 | butane | butyr = butter |
| 5 | pentane | penta = 5 |
| 6 | hexane | hexa = 6 |
| 7 | heptane | hepta = 7 |
| 8 | octane | octa = 8 |
| 9 | nonane | nona = 9 |
| 10 | decane | deca = 10 |
Functional Groups:
Click on the name of a functional group, then click on the
group in this picture.
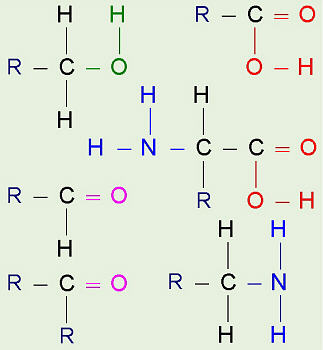 Alcohol (Hydroxyl Group)
Alcohol (Hydroxyl Group)
Aldehyde (Carbonyl Group)
Ketone (Carbonyl Group)
Carboxylic Acid (Carboxyl Group)
Amine (Amino Group)
Amino Acid (Amino Group + Carboxyl Group)
As just mentioned, generally “plain”
hydrocarbons are not found in in living cells. There are usually other
groups of atoms attached somewhere on the molecule. There are certain
groups of atoms that are frequently attached to the organic molecules we
will be studying, and these are called functional groups. These are
things like hydroxyl groups which form alcohols, carbonyl
groups which form aldehydes or ketones, carboxyl
groups which form carboxylic acids, and amino groups which
form amines. These groups tend to act the same and have similar
properties no matter where on a carbon backbone molecule they’re stuck.
Additionally, a molecule may have more than one functional group and/or more
than one type of functional group attached. For example, later, we will be
discussing glycerol, which is a propane with a hydroxyl group in place
of one of the hydrogens on each of the three carbons. Another example would
be amino acids, which have both both an amino group and a carboxyl
group attached. We will be discussing each of these functional groups in
more detail as we discuss the various types of molecules that contain
them.
Two Important Reactions:
As we look at the various types of molecules and how they are formed, we will
see two special reactions again and again in a number of situations. These
two reactions are exact opposites.
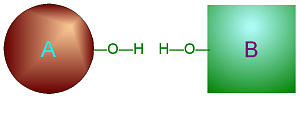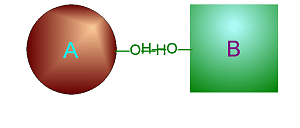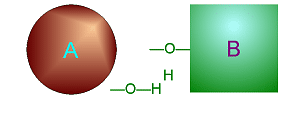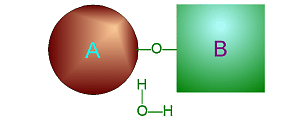
Dehydration Synthesis
involves taking water (one O and two H) out from two smaller molecules to
cause them to bond together to make one larger molecule.
Hydrolysis
involves adding water into a specific place in one large molecule to cause
it to break into two smaller ones.
Click on either of
these pictures to animate it.
| >>> Dehydration Synthesis <<< | <<< Hydrolysis >>> |
|---|
 |
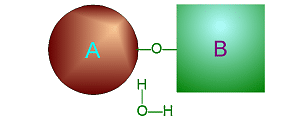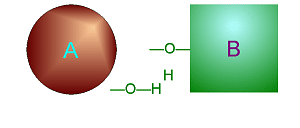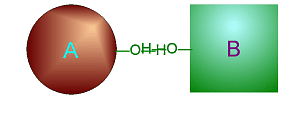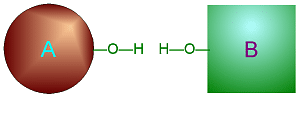 |
References:
Borror, Donald J. 1960. Dictionary of Root Words and Combining Forms. Mayfield Publ. Co.
Campbell, Neil A., Lawrence G. Mitchell, Jane B. Reece. 1999. Biology, 5th Ed. Benjamin/Cummings Publ. Co., Inc. Menlo Park, CA. (plus earlier editions)
Campbell, Neil A., Lawrence G. Mitchell, Jane B. Reece. 1999. Biology: Concepts and Connections, 3rd Ed. Benjamin/Cummings Publ. Co., Inc. Menlo Park, CA. (plus earlier editions)
Marchuk, William N. 1992. A Life Science Lexicon. Wm. C. Brown Publishers, Dubuque, IA.
Sienko, Michell J. and Robert A. Plane. 1966. Chemistry: Principles and Properties. McGraw-Hill Book Co., NY. (and other chemistry texts and handbooks)
Copyright © 1996 by J. Stein Carter. All rights reserved.
This page has been accessed  times since 15 Aug 2000.
times since 15 Aug 2000.



