Mitosis
2-D says, “When I arrived at Clermont College from
my parents’ home in the research lab in Kansas, I was only an embryo in
an egg, and look at me now! As an embryo, my body did lots of mitosis
to grow and develop. Finally, I hatched out of the egg and was a tiny
caterpillar. As I ate milkweed leaves, my body did more mitosis, until
I finally turned into a great big, “fat” caterpillar. After that, I
molted into a chrysalis, and my body went into high gear doing mitosis
that totally rearranged me into an adult butterfly. Mitosis is really
important stuff.”
Types of Cells and Replication of Chromosomes:
Cells can divide, and in
unicellular
organisms, this makes more organisms. In
multicellular
organisms, cell division is used for growth, development, and repair of the
organism. Cell division is controlled by
DNA,
but exact copies of the DNA must be given to the daughter cells (note use of
“mother” and “daughter”). Bacteria reproduce by a simple process called
binary fission. They have one chromosome which is attached to the
cell membrane. This chromosome replicates, then the two copies are pulled
apart as the cell grows. Eventually the cell pinches in two to make two
cells. Eukaryotes do
mitosis.
In mitosis, each daughter cell gets about half of the cytoplasm from the
mother cell and exactly one set or copy of the DNA.

Replicated Chromosome
Before cell division occurs, the cell first has to replicate the
chromosomes
so each daughter cell can have a set. When the chromosomes are replicated and getting ready to divide, they consist of two, identical halves
called sister chromatids which are joined by a central region, the
centromere.
Each chromosome is one long molecule of DNA and special proteins. DNA makes up the
genes,
and we say that genes are “on” chromosomes, or chromosomes “contain” or are made of genes. Some of the proteins in the chromosomes “turn off” the genes that are not needed in that cell. For
example, while every cell in your body contains exactly the same genes, you don’t need your eye-color gene operational in cells in your big toe, nor toenail-shape genes active in cells in your stomach.
Two basic types of cells occur in the bodies of eukaryotes.
Somatic cells
are general body cells. These have the same number of chromosomes as each
other within the body of an organism. The number of chromosomes in somatic
cells is consistent among organisms of the same species, but varies from
species to species. These chromosomes come in pairs, where one chromosome
in each pair is from the mother and one is from the father. Actually, since
most organisms have more than one pair of chromosomes, it would also be
correct to say that the organism received one set of chromosomes from
its mother and one matching set from its father, and that these sets match
in pairs. The other type of cells found in eukaryotes is
gametes
or sex cells, consisting of eggs in females and sperm in males. These
special reproductive cells have only one set (half as many) of chromosomes
consisting of one chromosome from each pair. In humans ONLY, the
somatic cells have 46 chromosomes arranged in 23 pairs (= two sets of 23
each), while gametes have 23 individual chromosomes (= one set). In fruit
flies, somatic cells have 8 chromosomes (= 4 pairs or 2 sets) and gametes
have 4 chromosomes (= 1 set). Geneticists use the term “-ploid” to
refer to one set of chromosomes in an organism, and that term is typically
combined with another wordstem that describes the number of sets of
chromosomes present. For example, a cell with one set of chromosomes is
called
haploid,
a cell with two sets of chromosomes is
diploid,
and a cell with four sets of chromosomes (not usually a “normal” condition,
but sometimes possible) is tetraploid. (I think I heard that our
domestic wheat is a hexaploid.)
Mitosis:
Technically, mitosis is specifically the process of
division of the chromosomes, while
cytokinesis
is officially the process of division of the cytoplasm to form two cells.
In most cells, cytokinesis follows or occurs along with the last part of
mitosis.
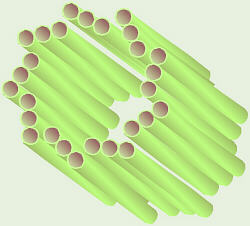
Centriole
Remember centrioles? They consist of nine sets of three microtubules, occur
in animal cells only, and are involved in division of the chromosomes.
Each animal cell has a pair of centrioles located just outside the nucleus.
The two centrioles in the pair are oriented at right angles to each other.
Just before mitosis, the centrioles replicate, so the cell now has four
(two sets of two) as it starts mitosis.
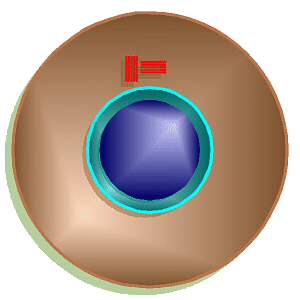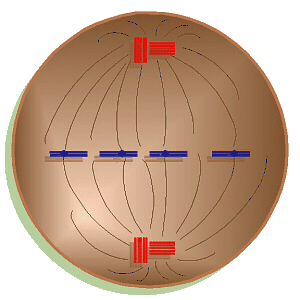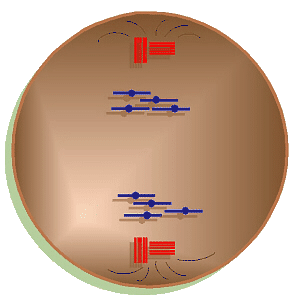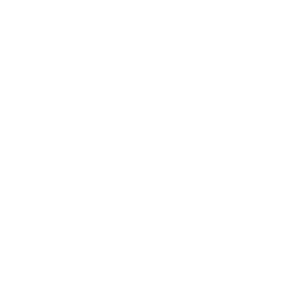
Mitosis Animation
The stages in mitosis include (interphase), prophase, metaphase, anaphase,
and telophase. Remembering “IPMAT” or Intelligent People Meet At
Three (or is that Twelve?) can help you remember the stages in
order. Strictly speaking, interphase is the stage in which a cell spends
most of its life and is not part of the process of mitosis, per se,
but is usually discussed along with the other stages.
Interphase
may appears to be a “resting” stage, but cell growth, replication of the
chromosomes, and many other activities are taking place during this time.
Near the end of interphase just before the cell starts into the other stages
of mitosis, if the cell is an animal cell, the centrioles replicate so there
are two pairs. At this time, the strands of DNA that make up the chromsosomes
are unwound within the nucleus and do not appear as distinct chromosomes.
Thus, at this stage, the genetic material is often referred to as
chromatin.
From here, the cell goes through all other stages of mitosis.

Mitosis in Onion Root Tip
In prophase,
the chromosomes start to coil, shorten, and become distinct. In animals only,
the centrioles begin to migrate to the poles of the cell. The mitotic
spindle or polar fibers begin to form from the poles of the cell
towards the equator. In animals only, this starts as
asters
around the centrioles. Eventually, the spindle mechanism finishes growing
toward the equator and interacts with the centromeres to line up and, later,
move the chromosomes. Also at this time, the nuclear envelope starts to
disintegrate.
Metaphase
is characterized by the lining up of the chromosomes along the equator of the
cell or what is called the metaphase plate. The nuclear envelope has
totally disintegrated and the polar fibers have reached the centromeres of
the chromosomes and have begun interacting with them.
In
anaphase,
the sister chromatids separate at the centromeres, thus can now be called
chromosomes. These are pulled to the poles of the cell by the mitotic
spindle.
In
telophase,
the new daughter nuclei and nuclear envelopes start to reform and the
chromosomes uncoil. Telophase frequently includes the start of cytokinesis.
In animal cells, cytokinesis starts with a cleavage furrow or
indentation around the middle that eventually pinches in, dividing the cell
in two. In plants, cytokinesis begins with a series of vesicles that form
at the equator of the cell, which subsequently join until the cell is
divided in two.
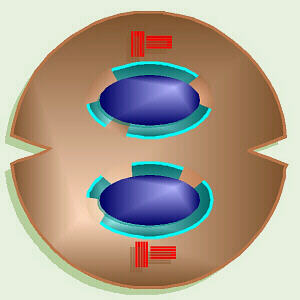
Animal Cytokinesis
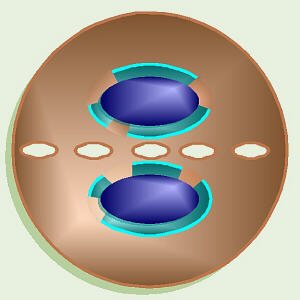
Plant Cytokinesis
Tissue Culture and Cancer:
Tissue Culture
One interesting offshoot of the study of mitosis is tissue culture.
In tissue culture, the cells to be studied are removed from the organism’s
body and grown on a sterile, artificial medium. When grown in this manner,
typically normal cells grow one layer thick on the surface of the sterile
medium and will undergo only 20 to 50 mitotic divisions then cease to be
able to reproduce (called the “Hayflick limit” after Leonard Hayflick, the
scientist who discovered it). Also, typically, when all cells are touching
neighbors
all around, they stop dividing. This phenomenon is known as contact
inhibition. In sharp contrast, cancer cells will not stop growing with
one layer on the surface of the medium, but grow multiple layers and fill
the dish. They do not exhibit contact inhibition: they don’t stop growing
when touching on all sides. Also cancer cells appear to have no limit to
the number of generations they can produce.
In January of 1951, back in the days of segregation, a poor,
young, black woman named Henrietta Lacks went to the designated “Colored”
area of Johns Hopkins Hospital to
be checked for a lump she could feel on her cervix. A biopsy showed that
she had cervical cancer, and so, in February, she went back to Johns
Hopkins for what, then, was the routine way cancer was treated: radium.
Without her knowledge, while she was anesthetized and before the radium was
inserted into her
cervix, samples of her cervical cancer were removed and sent to a researcher
named George Gey who was trying, unsuccessfully, to grow cells in tissue
culture (note: this was not related to any medical testing she needed,
but rather, merely to benefit his research).
Dr. Gey and his lab staff not only were able to successfully culture Ms.
Lacks’ cancer cells — the first cells to successfully be grown in tissue
culture, but also discovered that these cells were extremely
prolific, doubling in numbers every few days. As was customary in Dr. Gey’s
lab, those cell cultures were labeled with the first two letters of her
first and last names, HeLa. Unfortunately, those cells were also
extremely aggressive within Ms. Lacks’ body, and despite the radium treatments
along with x-ray treatments, they rapidly metastasized. By the following
September, she had huge tumors, many the size of baseballs, that had
practically taken over her body. Ms. Lacks
died on 4 October 1951, at an age of only 31 years old, survived by her
husband and young children, the youngest of whom was born just a couple
months before she first noticed that something was wrong.
In the mean time,
as was (and is) customary among researchers, Dr. Gey gave away samples of
the cells he was growing to many of his colleagues. In 1952, in the midst
of a huge polio epidemic, HeLa cells
were used to test Dr. Salk’s new polio vaccine prior to giving the vaccine
to children. Not long afterward, several of the biological supply companies
started to mass-produce and sell HeLa cells to the research community.
HeLa cells are now an extremely important research organism, used in
developing and testing of countless drugs that have saved many lives.
Ms. Lacks’ husband, children, and other relatives, struggling
to make ends meet in an inner-city Baltimore neighborhood, knew nothing of
this until reporters tracked them down and started asking them questions.
A number of her descendants had only a grade-school education, and not having
even high-school biology courses, were terrified to find out that, as it
sounded to them, the researchers were keeping their mother/grandmother
alive, somewhere, and experimenting on her. Ironically, while HeLa cells
are now a commonly-used research organism, worldwide, and a multimillion
dollar industry, many of her descendants are so poor and under- or
unemployed that they cannot afford health insurance.
In 2010,
Rebecca Skloot
published a biography of Henrietta Lacks, and has
established a
foundation
to use a portion of the proceeds of the book
to fund scholarships and medical expenses for Ms. Lacks’ descendants.
Within the past few years, an interesting issue has arisen
regarding these cells: can they still be considered “human”? While the HeLa
cells currently being grown in tissue culture are descendents of the
original human cancer cells, by now they have mutated so much, and there
are so many different “tissue lines” that are genetically different,
that some scientists have raised the question whether they can/should still
be considered “human” tissue, especially since they were abnormal, cancer
cells to begin with.
Tissue culture is now a widely-used means of more effectively
and quickly finding the right drugs to treat cancer. Typically, in the past,
people with cancer were subjected to one toxic drug after another in hopes of
finding one that would be effective against that particular cancer.
Unfortunately, by the time the right drug was found, it frequently was too
late to do any good. Now, when a person is diagnosed with cancer, a biopsy
can be taken and a number of cultures of cells can be grown. Each of these
cultures can be subjected to a different drug, thus enabling doctors to find
the right drug sooner, while it may still be of help, and without needlessly
subjecting the person to many kinds of toxic chemicals.
Now, we have a whole arsenal of cancer-fighting drugs. Many
of these drugs fit into one of three classes, based on their mode of action.
The “older” types of drugs typically work in one of two ways: they either
cause mutations in the person’s DNA, or they mess up mitosis, specifically
the mitotic spindle mechanism (the microtubules), in the person’s
body. The underlying concept behind both of these is that cancer cells are
rapidly dividing, so don’t have the time to repair damage caused by those
drugs, and therefore cease being able to divide, whereas most “normal” cells
divide more slowly and thus have more time to monitor for and repair any
DNA damage or damage to the mitotic spindle. That also explains why people
who are receiving chemotherapy typically lose their hair or develop
skin or mucus membrane sores: skin, mucus membrane, and hair follicle cells
are among some of the fastest-growing normal cells in our bodies.
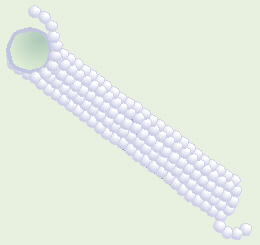
Microtubule Made of Tubulin
Vincristine is one frequently-used chemotherapy drug that messes up
the mitotic spindle. Microtubules are composed of a string of individual
protein molecules called tubulin. Vincristine binds onto/around the
tubulin, thereby inhibiting it from forming microtubules. That means that
rapidly-dividing cells, such as cancer cells, cannot make the mitotic spindle
(polar fibers) that they need in order to divide. Some of vincristine’s
side effects are due to the fact that microtubules also make up an important
part of the cytoskeleton in all cells, and vincristine inhibits formation
of all microtubules.
Some of the newest chemotherapy drugs on
the market work by
tagging the cancer cells as “bad guys” so that the person’s own immune
system can better identify them as foreign invaders and attack them.
Rituximab is one example. The “mab” part of its name stands for “monoclonal
antibody.” Here’s how it works. Many types of lymphoma cells have an
antigen (a chemical “ID tag”) called “CD-20” on their surface. Some
human lymphoma cells were injected into the bloodstream of mice, and as
would be expected, the mice’s immune systems started to produced
antibodies to fight the foreign-to-them CD-20 antigen. Next,
those mouse immune system cells were isolated and grown in tissue culture.
Then, since mouse and human immune systems are slightly different, those
cells were turned into GMOs (genetically-modified organisms) by hybridizing
them with human cells so that the anti-CD-20 antibodies they were producing
would be more like human antibodies. Also, cancer genes(!) were inserted
into them — not the cancer genes that would make someone get cancer, but
the gene(s) that enable cancer cells to overcome the Hayflick limit and
continue to grow “forever” in tissue culture. That way, huge numbers of
these GMO mouse-human hybrid cells can be grown in tissue culture, in a
“pure” clone of cells, all of which are producing, specifically, anti-CD-20
antibodies. Those antibodies are “only” proteins, which the cells secrete
into their growing medium, so after an appropriate amount of time, the
cells and their growing medium are separated, and the antibodies are isolated
from the medium, purified, and packaged for sale. When administered to a
lymphoma patient, these antibodies search for and tag any CD-20 antigens
they find in the person’s body, and when the person’s immune system “sees”
those CD-20 + anti-CD-20 complexes, it “knows” to destroy those cells. A
big advantage of this type of chemotherapy is that it is very specifically
targeted at the “bad guys,” and doesn’t cause the hair loss, skin problems,
etc., that are common with other chemotherapy agents.
Rate(s) of Mitosis
Within our bodies, different cells do mitosis at different
rates. Skin cells continuously do mitosis and divide, thus our skin is
constantly renewed and repaired. In sharp contrast, for a long time, it was
thought that most nerve cells stop doing mitosis soon after birth, but
we now know that, very slowly, nerve cells do divide.
(Caution: overconsumption of alcohol can kill nerve/brain cells, and they
cannot easily be replaced and “grow back.”).
Liver cells are somewhere in between. In a healthy adult, liver cells
normally do not divide, but can divide to repair minor damage. Major liver
damage or a disease like cirrhosis is too much damage to be repaired through
mitosis. In contrast, it is possible to use one adult liver to do liver
transplants for four babies, and if all goes well, these pieces can
eventually regenerate whole livers.
Note this comparison between mitosis and meiosis.
References:
Berkow, Robert, ed. 1999. The Merck Manual. 17th Ed. Merck, Sharp & Dohme, Rahway, NJ.
Borror, Donald J. 1960. Dictionary of Root Words and Combining Forms. Mayfield Publ. Co.
Campbell, Neil A., Lawrence G. Mitchell, Jane B. Reece. 1999. Biology, 5th Ed. Benjamin/Cummings Publ. Co., Inc. Menlo Park, CA. (plus earlier editions)
Campbell, Neil A., Lawrence G. Mitchell, Jane B. Reece. 1999. Biology: Concepts and Connections, 3rd Ed. Benjamin/Cummings Publ. Co., Inc. Menlo Park, CA. (plus earlier editions)
Marchuk, William N. 1992. A Life Science Lexicon. Wm. C. Brown Publishers, Dubuque, IA.
Skloot, Rebecca. 2010. The Immortal Life of Henrietta Lacks. Crown Publ. New York.

Copyright © 1996 by J. Stein Carter. All rights reserved.
This page has been accessed  times since 15 Aug 2000.
times since 15 Aug 2000.







