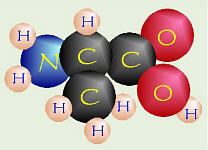
One Amino Acid Amino acids are built from a central carbon bonded to four different groups. (run your mouse over the amino acid to see the names of the parts)
2-D says, “While the nectar we butterflies drink contains lots of sugar, that’s actually not all we eat. Have you ever sipped orange juice through a straw? Even though it’s mostly liquid, it does contain some orange pulp, and if your straw is large enough in diameter, the pieces of pulp will go up it, too. The same is true for our soda-straw-like mouthparts. Even though much of what we consume is the nectar, we do also manage to ingest (eat) pollen from the flowers we visit. That might not be such a big deal for all those wimpy butterflies that die after only a few weeks as adults, but we Monarchs have to live long enough and have the endurance to fly all the way down to Mexico, and then live all winter down there before beginning the return trip. We typically don’t make it all the way back, but we ladies do lay lots of eggs before we die, and we need lots of protein to make those eggs. Pollen contains a lot of protein, and thus, that is a really important dietary source of protein for us.”
Proteins are about 50% of the dry weight of most cells, and are the most structurally complex macromolecules known. Each type of protein has its own unique structure and function.
Polymers are any kind of large molecules made of repeating identical or similar subunits called monomers. The starch and cellulose we previously discussed are polymers of glucose, which in that case, is the monomer. Proteins are polymers of about 20 amino acids (the monomer).

One Amino Acid
Amino acids are built from a central carbon bonded to four different
groups. (run your mouse over the amino acid to see the names of the parts)
In the approximately-20 amino acids found in our bodies, what varies is the side chain. Some side chains are hydrophilic while others are hydrophobic. Since these side chains stick out from the backbone of the molecule, they help determine the properties of the protein made from them.
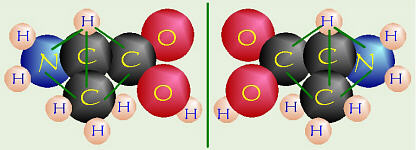
Left and Right Mirror-Image Molecules
Most naturally-occurring amino acids are the l- form, whereas synthetically-produced
amino acids give a 50:50 mixture. Notice that these molecules are mirror
images of each other, thus there is no way you can rotate one molecule to
make it look like the other.
To form protein, the amino acids are linked by dehydration synthesis to form peptide bonds. The chain of amino acids is also known as a polypeptide. Some proteins contain only one polypeptide chain while others, such as hemoglobin, contain several polypeptide chains all twisted together. The sequence of amino acids in each polypeptide or protein is unique to that protein, so each protein has its own, unique 3-D shape or native conformation. If even one amino acid in the sequence is changed, that can potentially change the protein’s ability to function. For example, sickle cell anemia is caused by a change in only one nucleotide in the DNA sequence that causes just one amino acid in one of the hemoglobin polypeptide molecules to be different. Because of this, the whole red blood cell ends up being deformed and unable to carry oxygen properly.
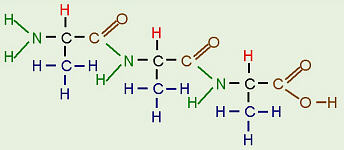
A Short Protein

Protein in Water
As a polypeptide chain forms, it naturally twists and bends into its native
conformation. One of the things that helps determine the native conformation
of a protein is the side chains of all the amino acids involved. Remember
some amino acid side chains are hydrophobic while others are hydrophilic.
In this case, the “likes” attract: all the hydrophobic side chains (here
represented by yellow beads) try to “get together” in the center of the
molecule, away from the watery environment, while the hydrophilic side
chains are attracted to the outside of the molecule, near the watery
environment. Additionally, some of the hydrophilic side chains have groups
of atoms attached that make them acidic, while others have groups attached
that make them basic. Side chains with acidic ends are attracted to side
chains with basic ends, and can form ionic bonds. Thus, the side chains
interacting with each other help to hold the protein in its native
conformation.

Protein in Organic Solvent
Denaturation
is when a protein loses its native conformation. For example, egg white
(also called albumen) contains a protein called
albumin
which is water-soluble. However, if heated, albumin becomes denatured and
loses its ability to be water-soluble. There are several possible things
that can denature proteins including changing the temperature (adding heat),
changing the pH or salt concentration of the solution, or putting the
protein into a hydrophobic (oily) solvent. In a hydrophobic solvent, the
amino acids with hydrophobic side chains (the yellow beads) would all try to
go to the outside of the molecule, and all those with hydrophilic side chains
would cluster in the center of the molecule. If a protein remains
water-soluble when denatured (unlike albumin), it can return to its native
conformation if/when placed back into a “normal” environment.
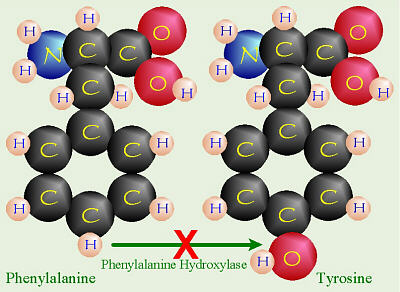
PKU: No Phenylalanine Hydroxylase
PKU or
phenylketonuria
is a genetic disorder for which, by law, all newborns must be tested.
People with this disorder are lacking an enzyme called phenylalanine
hydroxylase needed to turn the amino acid, phenylalanine into
tyrosine so it can be eliminated via the urine.
Since phenylalanine cannot be eliminated, it builds up and in children whose
nervous systems are still developing, that build-up causes mental retardation.
According to the Merck Manual, the body is very slowly able to turn it into
several other compounds which can be eliminated via the urine, but these
chemical reactions are too inefficient to get rid of the excess phenylalanine.
If PKU is detected shortly after birth, the child is put on a special diet
low in phenylalanine and has normal mental development. Since phenylalanine
is an amino acid that is needed by the body for normal growth and
development, the goal is to limit intake to just what the child needs without
any excess that could build up in his/her system. The Merck Manual says
that, unfortunately, most natural protein sources, including milk, are too
high in phenylalanine for children with PKU, so these babies/children must
be put on a special formula from which most, if not all, of the phenylalanine
has been removed. As the child grows, some low-protein, natural foods such
as fruits, vegetables, and some kinds of grain are OK to eat. The needed
phenylalanine is supplied via carefully-measured amounts of natural protein
plus the residual in the formula. One area of “debate” is how long the
special diet must be continued. Some people think the diet may be
discontinued after the brain finishes developing at about age 5. Others have
noted some behavior problems and learning difficulties that appear to be
related to going off the diet, and recommend that people with PKU stay on the
diet for life. Staying on the special diet is especially important if a
woman who has PKU is even thinking about trying to become pregnant. Because
any excess phenylalanine in her system could cross the placenta and could
cause mental retardation in the baby, especially in the first few weeks while
the nervous system is forming, she has to make sure that she adheres to the
special diet from before conception. Note that this is not a major concern
for a normal woman carrying a baby with PKU (she wouldn’t know that until
after the baby was born and tested) because the woman’s body can digest
phenylalanine, including any excess from the baby’s body that is sent back,
via the placenta, to her body.
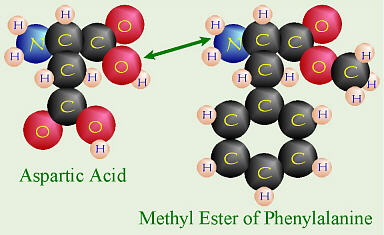
Formation of Aspartame (Nutrasweet®)
Nutrasweet® has the chemical name aspartame and is made of
two amino acids: l-aspartic acid and the methyl ester of
phenylalanine, thus people with PKU can’t have it. Look at a can of
diet soft drink that is sweetened with aspartame. Somewhere on the can,
there’s always a cautionary label warning people with PKU not to drink it.
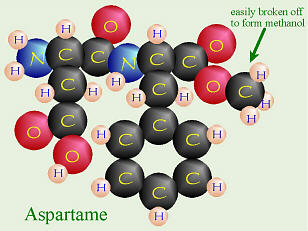
Aspartame (Nutrasweet®)
But what about the rest of us? That methyl group on the phenylalanine can
break off pretty easily, and at temperatures as “low” as 86° F (= 30° C —
lower than body temperature or a summer day), aspartame starts to
disintegrate, releasing methanol (which in larger quantity causes blindness,
etc. — it’s toxic). As the liver tries to deal with the methanol using
normal metabolic pathways, it ends up turning the methanol into formaldehyde,
which is a known carcinogen. Interestingly, because of this and several
other concerns, originally there was controversy over FDA approval of
aspartame. Initially, due to the concern over the fate of that methyl group,
it was only approved for use in cold food items (not cocoa or cake mixes),
but with industry pressure, that soon changed. Even still, its safety is
controversial. More recently, some people have suggested a possible link
between aspartame consumption and an increase in brain tumors in this
country.
Equal® is not the same as Nutrasweet, but does contain some of it. There’s an interesting psychological thing here: in our culture, people think they need sweetener by the teaspoon, but Nutrasweet is so much sweeter than sugar that only a tiny pinch would be needed to gain the equivalent sweetness. Artificial sweetner manufacturers know that if they don’t make a product such that one teaspoon is as sweet as one teaspoon of the sugar people are used to using, people will use one teaspoon anyway (a tiny pinch just doesn’t look like enough), then say it was too sweet and stop buying it. Thus, artificial sweetners are designed to take up about a teaspoon of space, and Equal® is mostly glucose (which diabetics and hypoglycemics shouldn’t have) with a little Nutrasweet added, so in theory, not quite as much of the sugar is needed (also remember that glucose isn’t as sweet-tasting as sucrose). To make the sweetness of one teaspoon of sweetner powder equivalent to that of one teaspoon of sucrose, various fillers (things like cellulose) are added. These products also contain a number of other chemicals, including silicon dioxide (SiO2) “to prevent caking.” This is the chemical name for sand or glass (although, from what I’ve heard, a bit of silicon is needed for healthy fingernails)! Pre-mixed, pre-packaged items, such as artificially-sweetened soft drinks, can contain all aspartame as their sweetener because the manufacturers of these products do not have to be concerned with the “one teaspoon” psychological factor. Remember, because aspartame degrades into methanol at warm temperatures, it should not be used in anything hot or anything that will be cooked (coffee sweetener, cake mixes, cocoa, etc.), and bottles of artificially-sweetened soft drinks that have been sitting out in the hot sun at the local gas station are suspect.
Berkow, Robert, ed. 1999. The Merck Manual. 17th ed. Merck, Sharp & Dohme, Rahway, NJ.
Borror, Donald J. 1960. Dictionary of Root Words and Combining Forms. Mayfield Publ. Co.
Campbell, Neil A., Lawrence G. Mitchell, Jane B. Reece. 1999. Biology, 5th Ed. Benjamin/Cummings Publ. Co., Inc. Menlo Park, CA. (plus earlier editions)
Campbell, Neil A., Lawrence G. Mitchell, Jane B. Reece. 1999. Biology: Concepts and Connections, 3rd Ed. Benjamin/Cummings Publ. Co., Inc. Menlo Park, CA. (plus earlier editions)
Marchuk, William N. 1992. A Life Science Lexicon. Wm. C. Brown Publishers, Dubuque, IA.
Copyright © 1996 by J. Stein Carter. All rights reserved.