Yeast Plate Count Lab
Making a Serial Dilution
Robert Koch, Single-Colony Isolation, and Koch’s Postulates
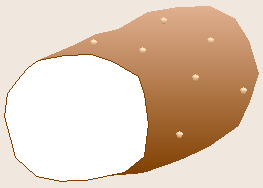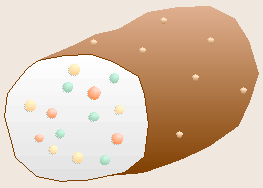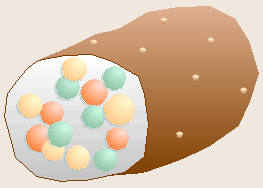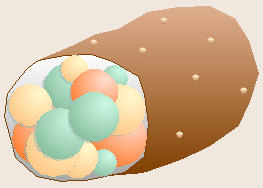 Robert Koch, a German physician, is famous for determining the bacteria
responsible for anthrax and tuberculosis.
The chance observation of bacteria growing on the surface of a spoiling slice
of boiled potato led Koch to realize that each spot, or colony, of bacteria
had grown as a clone from a single contaminating bacterium that had
previously landed on the potato. From this realization, Koch developed the
technique called single colony isolation, in which a sample of
bacteria from one colony may be used to inoculate a culture medium and from
that, grow a pure culture of that species of bacterium.
Robert Koch, a German physician, is famous for determining the bacteria
responsible for anthrax and tuberculosis.
The chance observation of bacteria growing on the surface of a spoiling slice
of boiled potato led Koch to realize that each spot, or colony, of bacteria
had grown as a clone from a single contaminating bacterium that had
previously landed on the potato. From this realization, Koch developed the
technique called single colony isolation, in which a sample of
bacteria from one colony may be used to inoculate a culture medium and from
that, grow a pure culture of that species of bacterium.
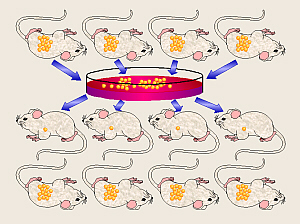 In his work with bacteria, Koch also developed a series of methods for determining
which species of bacterium is responsible for a given disease. In his honor,
these four steps are collectively known as Koch’s Postulates.
These say that to prove that a given bacterium causes a certain disease, the
researcher must:
In his work with bacteria, Koch also developed a series of methods for determining
which species of bacterium is responsible for a given disease. In his honor,
these four steps are collectively known as Koch’s Postulates.
These say that to prove that a given bacterium causes a certain disease, the
researcher must:
- find the same bacterium species in all diseased
individuals investigated,
- isolate the bacterium from diseased individuals and
grow it in a pure culture,
- induce the disease in experimental animals by
transferring bacteria from the culture, and
- isolate the same bacterium from the animal after the
disease develops.
Koch and other researchers at that time were also looking for
a suitable medium upon which to grow bacteria for study. Gelatin was ruled
out as a solidifying agent because (as any picnic-goer knows) a mixture of
gelatin and water is not solid at body temperature (37° C). Also, because it
is a polypeptide, it is digested by a number of species of bacteria, thereby
losing its gelling ability. A housewife friend suggested that Koch try agar,
noting that many cooks used it to solidify desserts, etc. in place of gelatin.
Koch subsequently developed the use of agar, a sulfuric ester of a
polysaccharide (poly = many, sacchar = sugar) complex derived
from certain red algae (Japanese Isinglass, Gelidium spp., is the best,
but also derived from other genera — obtained by boiling the seaweed for 6 hr
in dilute H2SO4), to solidify nutrient liquid media.
This allowed the growth on the surface of the medium of a specimen spread
across it, thus isolating the various microorganisms (micro = small)
which might be present. Agar was found to be ideal as a gelling agent — it
melts/dissolves only when the media are heated to near boiling, will remain
melted until cooled to around 40° C, and forms a gel that stays solid at body
temperature. Because it is a complex polysaccharide, it is not degraded by
the vast majority of bacteria, thus the medium remains solid and does not
liquify as the bacteria grow in/on it, and therefore allows strict control of
growth factors which may be limiting.
We Will Use Yeast
In this experiment, we will be using yeast (a fungus and
something which is a lot safer for students who are just learning sterile
techniques to handle) rather than bacteria to learn some of the techniques
and methods used in microbiology. We will be making use of Koch’s conclusion
that each viable cell (in this case yeast) that happens to land on the medium
can/will grow into a small colony that we can see and count.
We will be
inoculating
4% glucose medium with an unknown number of yeast cells, and like Koch’s
conclusion that each bacterial colony on the potato came from a single, live
bacterium, we will assume that each yeast colony we find growing on our agar
plates came from a single, live yeast (a
colony-forming unit or CFU).
We will use this information to calculate the average number of yeast cells
in a packet of yeast.
However, there are too many yeast cells in a packet to count
them all, so we must dilute them. If we keep track of how much they were
diluted, we can use the number of colonies growing on our plates to “work
backwards” to calculate the number of cells in a yeast packet. Because the
yeast must be very dilute to get countable results, we need to do a serial
dilution and to spend time discussing the math involved in figuring out
the number of cells per packet.
Experiments by Dr. Fankhauser’s Microbiology students have
shown that yeast, Saccharomyces cerevisiae (myce = fungus,
Ceres = goddess of grain, visi = look or see), grows optimally
on a nutrient agar supplemented with 4% glucose (gluco = sweet,
ose = sugar or carbohydrate). Remember that glucose and dextrose
(dextro = right) are just two names for the same thing. This medium
contains nutrient broth which consists of 0.3% beef extract, and 0.5% of a
pepsin digest of beef (peptone). It thus contains a broad variety of amino
acids and vitamins providing a suitable medium for a wide variety of
non-fastidious microorganisms (fastidious ones have very complex, specific
nutritional requirements). The 4% glucose content especially encourages the
growth of yeast with its ability to ferment at high rates. Thus, that is
the medium we will be making for our yeast.
First Day — Preparation of Medium
For this lab, work in groups. A batch/bottle of medium
will make about 20 to 25 agar plates. Thus, making two batches/bottles
per lab section of 15 to 20 people should suffice.
If not already present, assemble the necessary equipment
and ingredients.
- 6 g nutrient broth powder
- 9 g agar-agar
- 24 g glucose (dextrose)
- (3 g NaCl, opt.)
- 600 mL dH2O
- balance
- spoons or spatulas
- 1000 mL beaker
- 1-L bottle with cap
- funnel
- heat source (microwave or pot of boiling water on the stove)
- hot pads
- (spirit thermometer, 10 to 110° C, opt.)
- autoclave
- 16 to 20 sterile petri dishes
- incubator
Weigh out the dry ingredients.
- Weigh the 1000-mL beaker, and record
its apparent weight. If it’s too heavy for the balance, counterbalance it
with an equal-sized beaker on the other balance pan.
- In your lab notebook, add the
required mass of the first reagent to the apparent weight of the beaker, and
then set the balance to read that weight.
- Add that dry reagent to the beaker
with care until equal balance swings are achieved. Gently tap on the edge of
the beaker with the spatula/spoon to better judge how “close” you are. If you
go slightly over the required amount, do not remove any excess back to the
original container. If/when you are weighing the nutrient broth powder,
replace the cover immediately on the nutrient broth container because it is
hygroscopic (hygro = moist or wet, scope = see or watch
or look; it absorbs moisture from the air and “turns into a rock,” like brown
sugar does in the summer). Move the balance weights to find the actual weight
added and record that in your lab notebook.
- For each of the other dry ingredients,
again, as in the last two steps, add the new balance reading plus the required
mass of the next reagent, then set the balance to read that weight. Weigh
that ingredient into the same beaker along with the the previous ingredient(s),
which does necessitate careful attention as you reach each “end point”
because you cannot remove excess if you accidentally add too much.
Mix and autoclave the medium.
- Add dH2O to the beaker
while stirring, and q.s. to 600 mL. Continue stirring until there
are no lumps and the dry ingredients are thoroughly suspended/dissolved.
- Use a funnel to transfer the medium
to a 1-L bottle. Note that, at this point, the agar is not melted/dissolved
in the medium and will settle out if given a chance. However, it is
important to get it all as you transfer the medium to the bottle. Thus, it
is necessary to swirl/stir the medium as it is being poured to help insure
that all the agar gets poured into the bottle along with the other
ingredients.
- Heat the medium to melt/dissolve
the agar. There are several ways of doing this – pick one.
The “traditional” method is to place the bottle in a pot of boiling water on
the stove, and while stirring with a thermometer (thermo = heat,
meter = to measure), heat to boiling, but do not allow it to
boil over, nor to burn on the bottom. A more “risky” method is to leave
the mixture in the 1000-mL beaker, and set that directly on the stove or
over a Bunsen burner to heat, while stirring with a thermometer (then, pour
into the bottle after it’s heated). A “quick, modern” method is to microwave
the medium: heat 2 min., swirl, heat 2 min, swirl, heat 1 min. No matter
which method you use, have hot pads available, and be prepared to quickly
turn off the heat source and then remove the bottle, if needed, to prevent
it from boiling over.
- Cap the bottle loosely, (if needed –
if the medium will be stored in the bottle, label the bottle with the group
name and date and/or apply autoclave tape to the lid), and place in the
autoclave. When both groups have put their bottles in the autoclave,
autoclave at 15 lbs. pressure for 15 min. (refer to autoclave protocol for
how-to). Although set for 15 min, this process will take around 45 min
to complete.
- When the autoclave is done and the
pressure is almost back down to zero, optionally use the stove to heat a pot
of water to between 50 to 60° C. This will be used to cool the autoclaved
bottles. If it’s cooler than 50° C, you risk having the bottles crack, and
if it’s warmer than 60° C, it won’t cool the bottles as effectively. However,
for the safety of the bottles, closer to 60° is better than closer to 50°.
- When the autoclave pressure has
returned to zero, carefully open the autoclave. Using hot pads, remove the
bottle(s) and let them cool at room temperature for at least several minutes.
After that, to speed the cooling process, the bottles may carefully be put
into the warm water. Ideally, the bottles should be cooled enough that
they feel “hot” but are just cool enough to be hand-held.
Sterilize a work area and pour the plates.
- On one of the lab tables, attach
Bunsen burners to the desk stopcocks. Obtain a striker, a squirt bottle of
70% ethanol, a scissors and a sealed bag of plastic petri dishes for each
bottle of medium that has been made. Use the alcohol and a paper towel to
“sterilize” an area of the desktop. Then, working on the cleaned area of
the table, cut the top off the bag of petri dishes, invert the bagged dishes,
and slowly and carefully, slide the bag up, off the petri dishes (save the
bag for future use). After the petri dishes are out of the bag, they may be
re-stacked in convenient-height stacks of about 3 or 4 dishes each, cover-side
up. Remember, these petri dishes are sterile inside, and need to remain
sterile inside. Do not open them up.
- Working on the sterile field, each
student should pour a stack of two to three plates using sterile technique
as demonstrated by your instructor (your group/class should use up all
the medium in your bottle — a total of 20 to 25 plates). To make it easier
to pour the plates, stacks of 3 or 4 sterile plates should be positioned near
the edge of the desk. Remove the cap from the bottle and hold it with the
little finger of the hand that’s holding the bottle (do NOT set it down on the
desk). Flame the lip of bottle to sterilize it.
- Starting with the bottom
plate in your stack, use your other hand to lift that plate’s lid and the
rest of the stack of plates above it straight up. Do not set these down on
the table, and do not hold them to the side of the plate that’s being filled.
Rather, hold them straight above the plate being filled as a “shield” to
help protect that plate from airborne bacteria falling into it. Fill each
plate about half full.
- After all the plates in that stack
have been poured, flame the lip of the bottle, and loosely replace the cap
(until the next person pours his/her plates). Carefully slide your stack of
plates to an open area of the desktop and to help them cool more quickly,
carefully unstack them so each plate is resting directly on the desktop.
If all the plates in your bag have been used up, and there is still some
medium in the bottle, look for plates that are a bit “low” — that contain
a bit less medium than the others — and top them off.
- When all the plates have been poured
and all the medium in the bottle has been used up, extinguish the Bunsen
burner and immediately rinse the bottle with hot water before any left-over
medium in it has a chance to gel. Any sterile, unused plates may carefully
be returned to their plastic bag.
- When the plates have cooled and
solidified, invert them, place them in a dish tub (or other, similar-sized
container), tape a label on the tub/container with the lab section,
instructor’s name, and date, then place into the 37° C incubator. Incubate
the plates for 48 hr to check for sterility.
Second Day — Serial Dilution and Inoculation of Plates
If not already present, assemble the necessary equipment
and ingredients.
- per student:
- test tube rack to hold 16×150-mm (large) test tubes (from under
the sink)
- on first pair of lab tables (repipet stations):
- rack with capped, sterile, 16×150-mm (large) test tubes (at least
3 tubes per student)
- 2 wax pencils
- 2 repipets with sterile dH2O, set for 9.9 mL
- 2 Bunsen burners
- at least 1 striker
- squirt bottle of 70% EtOH
- on second pair of lab tables (serial dilution stations):
- 250-mL beaker containing 100 mL dH2O, on magnetic
stirrer (note: need one 100 mL of water per class, so the other
may, temporarily, be empty)
- unopened packet of fresh bakers’ yeast (1 per class)
- canisters of sterile 0.1-mL pipets (at least 3 pipets per
student)
- at least 2 pipet bulbs or pipet fillers
- 2 Bunsen burners
- at least 1 striker
- used pipet container
- vortex
- squirt bottle of 70% EtOH
- on third pair of lab tables (spectrophotometer stations):
- spectrophotometer set at 660 nm
- lens paper
- set of 2 clean, matched cuvettes (one containing dH2O)
in plastic test tube rack (“small” test-tube size)
- on fourth pair of lab tables (plating-out stations):
- 2 turntables
- 2 spreaders, each in a beaker with 95% EtOH
- canisters of sterile 1.0-mL pipets (at least 1 per student)
- at least 2 pipet bulbs or pipet fillers
- 2 Bunsen burners
- at least 1 striker
- used pipet container
- 2 wax pencils
- squirt bottle of 70% EtOH
- on fifth pair of lab tables:
- sterile 4%-glucose nutrient agar plates made last lab period
- plastic “dish” tub into which to put inoculated plates
- other:
Get ready to do the lab.
- At the second station, your
instructor will suspend the contents of a package of yeast in 100 mL of water.
This willbe mixed thoroughly (using a magnetic stirrer) for at least
5 to 10 min. If stations are set up on both sides of the room, your
instructor may divide the yeast suspension between two beakers and place one
on each magnetic stirrer.
- If you haven’t already done so,
obtain a “big” test tube rack from under the sink.
At the first table (repipet station):
- Obtain three, sterile, capped,
16×150-mm test tubes and use the wax pencil
to label them “2,” “4,” and “6” to represent the three serial dilutions
(10–2, 10–4, and 10–6) you will be
making.
- Check to insure that the repipet is
set for 9.9 mL.
- Obtain or share one paper towel.
Squirt some of the 70% EtOH onto the
table top, and wipe to sterilize it.
- As demonstrated by your instructor,
remove the lid from a test tube and
hold it with your little finger — keep the cap off the tube the minimum time
necessary and do not set it down. Flame the lip of the tube. Raise the
plunger on a repipet to fill it, then push down to deliver 9.9 mL of sterile
dH2O into that tube using a repipet.
- Again, flame the lip of the test tube,
and replace the lid.
- Repeat for your other two tubes.
At the second table (serial dilution station):
- Begin with a sterile field by using
a paper towel (obtain or share) to wipe the desktop with 70% EtOH (95% will
dehydrate the bacteria rather than being absorbed to kill them). It is
necessary to do this once at the beginning of the lab, but use your judgement
if you think it needs to be done again (if someone leans on the table).
Squirt some of the 70% EtOH onto the table top, and wipe to sterilize
it.
- Make sure you understand how the
pipet filler works and how to use it before you start working with
sterile equipment.
- Perform a 106 serial
dilution of the yeast suspension as follows. So that you get “good” results
and your plates don’t get contaminated, it is very important to not rush
through this, but rather, to concentrate on what you are doing.
This procedure is extremely important in microbiological labs, and is one of
the crucial techniques in aseptic (a = not or without, septi =
rotten or putrid) technique. While the steps may seem overly detailed in the
following narrative, care in learning proper technique at the beginning
establishes good technique for the rest of your life. Compare these detailed
steps with the demonstration given by your instructor. Patience pays off.
Go slowly at the beginning, and verbally (not physically) assist your fellow
students as they work through the steps.
- Do the first dilution:
- So that your dilutions don’t become “contaminated” by droplets
of more-concentrated yeast solution, you must use a fresh 0.1-mL pipet
each time.
- Out of the 100 mL of yeast suspension that was made, you will use
0.10 mL to do the serial dilution. Note that this 0.10 mL contains
1/1000 or 103 of the yeast in the original packet
(0.10/100 = 1/1000).
- With your rack of sterile test tubes right there and “ready-to-go,”
obtain a sterile, 0.10-mL pipet from the canister. Only touch the pipet
you are withdrawing, and only touch it by the top end — it is sterile,
and touching it will transfer bacteria from your hands and make it
non-sterile.
-
- Pass the tip end of the pipet through the Bunsen-burner flame to
make sure it is sterile. Fit the pipet into the end of a pipet
filler.
- Using your writing hand, use the pipet and pipet filler to obtain
0.10 mL of the yeast suspension from the beaker on the magnetic stirrer.
Use caution because the 0.01-mL pipets are “tiny” and will fill up a
lot more quickly than the 5.0-mL and 1.0-mL pipets you’ve used
before.
Then, tilt the pipet slightly horizontally so that fluid moves up
slightly into the pipet and doesn’t drip during transfer.
- Without delay, and without touching anything with the pipet, pick
up the first (“2”) test tube with your non-writing hand, grip the cap
with the little finger of the pipet hand and gently remove with
twisting-pulling motion. Hold the cap in that little finger and do
not lay it down.
- Flame the lip of the test tube, then if needed, set the test tube
back in the rack. Deliver the 0.10 mL of yeast suspension into the
test tube, remembering to puff out the last drop.
- If needed, pick up the test tube. Reflame the lip of the test
tube. Replace the cap and return the test tube to the rack. Do this,
first, as soon as possible, before dealing with the pipet, etc.
- Remove the pipet from the pipet filler, and place it in one of the
used-pipet containers.
- Mix the contents of the test tube well with a vortex. Note that
this means you have used that 0.1 mL of solution to make 10 mL of
solution — 100 times as much, thus the concentration is 1/100
(10–2) as strong as it was before.
- Do the second dilution:
- Obtain a new, sterile 0.10-mL pipet. As before, pass the tip end
of the pipet through the Bunsen-burner flame to make sure it is
sterile. Fit the pipet into the end of a pipet filler.
- Without delay, and without touching anything with the pipet, pick
up the first (“2”) test tube with your non-writing hand, grip the cap
with the little finger of the pipet hand and gently remove with
twisting-pulling motion. Hold the cap in that little finger and do
not lay it down.
- Flame the lip of the test tube, then if needed, set the test tube
back in the rack. Use the pipet and pipet filler to obtain
0.10 mL of the yeast suspension from the first test tube.
Use caution because the this solution is more clear than the previous
one, and is very difficult to see as it rises (quickly!) in the
pipet.
- If needed, pick up the test tube. Reflame the lip of the test
tube. Replace the cap and return the test tube to the rack.
- Without delay, and without touching anything with the pipet, pick
up the second (“4”) test tube, remove the cap, and flame the lip of
that test tube, then if needed, set the test tube back in the rack.
Deliver the 0.10 mL of yeast suspension into the test tube.
- Reflame the lip of the second test tube. Replace the cap and
return the test tube to the rack. Then, remove the pipet from the
pipet filler, place it in one of the used-pipet containers, and
mix the second test tube with the vortex.
- You now have a solution that is 100 × 100 = 10,000 (104)
times as dilute. Note that the 0.10 mL used to make this dilution
contains 10–3 × 102 = 105 of the
yeast in the original packet.
- Do the third dilution:
- Again using sterile technique and a new, sterile pipet,
use this same procedure to transfer 0.10 mL from the second test
tube (“4”) to the third (“6”) one. Remember to mix this tube with
the vortex, too.
- Notice that you now have a solution that is 100 × 10,000 = 1,000,000
(102 × 104 = 106) times as dilute.
Also, the 0.10 mL used to make this dilution contains
10–5 × 10–2 = 10–7 of the yeast in
the original packet.
At the third table (spectrophotometer station):
- Check to make sure the wavelength of
the spectrophotometer is set to 660 nm. Use the cuvette of dH2O
to blank the spectrophotometer.
- In the other cuvette, pour some of
the contents of your first (“2”) tube only (assuming you did your
dilutions correctly, you’re done with that tube, now). Note that this
solution is no longer sterile, so make sure you do not mistakenly use the
solution in your third tube!
- Use the spectrophotometer to
determine the A660 of the 102 dilution and record that
number in your lab notebook. Since everyone is working from the same initial
yeast solution and everyone is doing the same dilutions, that means everyone’s
absorbance readings should be about the same, so this step is just to
double-check.
At the fourth table (plating-out station):
- As before, obtain or share one paper
towel. Squirt some of the 70% EtOH onto the table top, and wipe to
sterilize it.
- Obtain two, sterile, 4%-glucose
nutrient-agar plates from the fifth table. Pay attention to what you’re
doing, and make sure you take your plates from the stack of sterile plates,
and not some that someone else has already inoculated. Hold your chosen
plates up to the light and examine them to verify that they are sterile
and nothing is growing on them.
- Label the bottom of each
of your plates in small letters (you’ll need to be able to see through
the plate) with the date, your initials, and aliquot size (0.2 or
0.1 mL).
- Use a 1.0-mL pipet to plate out
0.10 and 0.20 mL of the 106 dilution onto the corresponding
plates.
- Obtain a sterile 1.0-mL pipet from the canister. As above, flame
the pipet and fit it into a pipet filler.
- Again using your little finger to hold the lid, and without
touching the pipet to anything, remove the lid of your third (“6”)
test tube, and flame the mouth of the test tube.
- Draw at least 0.3 mL of liquid into the pipet, and adjust the
level to a convenient 0.10-mL marking.
- Reflame the mouth of the test tube and replace its cap.
- Working quickly and accurately (so all of your sample doesn’t end
up in one spot on the plates), from that pipetful of liquid, release
0.1 mL into the “0.1 mL” plate and 0.2 mL into the “0.2 mL”
plate.
- Place the pipet into the used-pipet
receptacle. Place the 0.1 mL plate onto the turntable. You want to do that
one first since it contains less liquid, and therefore is slightly more likely
to have all the liquid absorb into one spot unless you work quickly.
- Obtain a spreader and shake off the
excess EtOH. When it is no longer dripping, pass the spreader briefly
through the flame of a Bunsen burner to ignite the rest. DO NOT HOLD
A FLAMING SPREADER OVER THE BEAKER OF EtOH!
- When the flaming has stopped, slightly
lift the lid of the 0.1-mL petri dish and touch the spreader to the inside of
the top of the petri dish to cool it. Hold the lid of the petri dish over
the dish to shield the dish from possible airborne contamination, and with
that same hand, slowly turn the turntable. With your other hand, hold the
spreader so it skims across the surface of the agar to spread your sample.
Do not press down on the spreader or you might damage agar surface. Again,
during this process, hold the petri dish lid above the plate to reduce
airborne fallout contamination. When the fluid has been absorbed, the
spreader will drag with a bit more difficulty, so you know that sample is
“done.”
- Place the spreader back into
the 95% EtOH.
- Remove the 0.1-mL plate from the
turntable and place the 0.2-mL plate on the turntable.
- As above, flame the spreader, then
spread the second plate.
- Once both of your plates are “done,”
invert both plates (agar side up), and place in the designated container to
be incubated at 37° C for 48 hr (2 days).
The plates should be stored agar-side-up in the incubator so any condensation
that is formed will be absorbed back into the agar rather than dripping onto
the plate and “messing up” your colonies.
- Note that since you are using only
0.1 mL (or 0.2 mL) out of the 10 mL in the last dilution, the 0.1 mL spread
on the first plate contains 10–7 × 10–2 = 10–9
of the yeast in the original packet (and the 0.2-mL sample, thus, contains
twice as much, or 2 × 10–9 of the yeast from the packet).
Third Day — Counting Yeast Colonies
If not already present, assemble the necessary equipment
and ingredients.
- petri plates from last period
- wax pencil and/or clicking counter
- opt. colony counter or other light source
Count the number of colonies on each of your plates. In
theory, the 0.20-mL plate should have approximately twice the colonies as
the 0.10-mL plate. Record these numbers in your lab notebook.
Assuming that one yeast cell can grow to form a colony of
yeast as it grows and divides (it is a colony-forming unit), calculate the
number of colony-forming units (CFU) in the original yeast package
Note that the 0.1-mL plate is 10–9 as concentrated as the original
package. The 0.2-mL plate is 2 × 10–9 as concentrated as the
original package. Thus, the package of yeast would contain
109 × [number of colonies on the 0.1-mL plate] or
5 × 108 × [number of colonies on the 0.2-mL plate] CFUs (actual
yeast cells).
Once you have gathered all your data and completed all your
calculations,
submit your data
online. Once everyone has submitted their data, you may print out a copy of
the
class data
for your lab notebook.
Dr. Fankhauser’s Dilution Practice Problems
In your lab notebook, do the dilution practice problems
which follow.
Because solutions in science are often much more concentrated
than are desired or can be managed for a given protocol, it is frequently
necessary to dilute these solutions. This requires a working knowledge of
the principles of diluting, dilution factors, concentration factors and the
calculations involved. High dilutions are usually expressed
exponentially.
First, some definitions:
- Aliquot:
- a measured sub-volume of sample
- Diluent:
- material with which the sample is diluted
- Dilution factor:
- ratio of final volume (aliquot plus diluent volume) divided by
the aliquot volume
- Concentration factor:
- ratio of aliquot volume divided by the final volume
Example: You make a dilution by adding 0.1 mL specimen
to 9.9 mL of diluent which gives a final volume of 10 mL:
- Dilution Factor = final volume/aliquot volume =
(0.1 + 9.9)/0.1 = 1 to 100, 1:100 or 102
- Concentration Factor = aliquot volume/final volume =
0.1/(0.1 + 9.9) = 0.01 or 10–2
To prepare a desired volume of solution of a given
dilution:
- Calculate the volume of the
aliquot:
aliquot
volume = concentration factor × final volume
- Calculate the volume of the
diluent:
volume
of diluent = (final volume - sample aliquot volume)
- Measure out the correct volume of
diluent, add the correct volume of aliquot to it, mix.
SAMPLE PROBLEMS:
- How much sample is required to
prepare 10 mL of a 1 to 10 dilution, and how much diluent would you need?
- What is the dilution factor when
0.2 mL is added to 3.8 mL diluent? What is the concentration factor?
- What should the aliquot and diluent
volumes be to prepare 5 mL of a 102 dilution?
- You have 0.6 mL of sample, and want
to dilute it to a fiftieth of its present concentration. How much diluent
will you add, and what will the final volume be?
- How would you prepare 20 mL of a
1:400 dilution?
- What is the dilution factor when you
add 2 mL sample to 8 mL diluent?
- You want 1 L of 0.1 M NaCl, and
you have 4 M stock solution. How much of the 4 M solution and how much
dH2O will you measure out for this dilution?
- You add a pint of STP gas treatment
to a 12-gal. fuel tank, and fill it up with gas. What is the dilution
factor?
- You diluted a bacterial culture
106, and plated out 0.2 mL and got 45 colonies on the plate. What
was the concentration of bacteria in the original undiluted culture?
- A hard one: You have 100.0 mL of
dH2O. How much glycerine would you have to add in order to make a
2.00% v/v (volume per volume) solution of the glycerine? (Hint: it requires
a little algebra.)
- Here’s another “English system” one
(for people who aren’t interested in cars and STP?): if you are making
homemade ice cream, and put 1’tsp of vanilla in a 1-gal. batch of ice cream
mix, what is the dilution factor?
(The answers are on the data-submission Web page.)
Other Things to Include in Your Notebook
Make sure you have all of the following in your lab notebook:
- all handout pages, including the dilution practice problems (in notebook or separate protocol book)
- all notes you take during the introductory mini-lecture
- all notes and data you gather as you perform the experiment
- all requested calculations based on those data
- print-out of class data (available online)
- drawings (yours!) of all “new” equipment used, including:
- autoclave (also cross-referenced to autoclave protocol)
- bottle used to autoclave the agar
- petri dish
- repipet (detail of scale set for 9.9 mL)
- magnetic stirrer/hot plate
- stir bar
- 16×150-mm test tube(s) with cap(s)
- 0.1-mL pipet
- turntable
- spreader
- clicking counter
- colony counter (detail of grid)
- incubator (including controls)
- drawing or xerox of your “finished” plate the day we count the
colonies
- optional sample of yeast packet
- answers to all discussion questions, a summary/conclusion in your
own words, and any suggestions you may have
- evidence that you have at least tried to work the practice
problems (not just answers, but actually showing your work)
- any returned, graded pop quiz
Copyright © 1996 by J. Stein Carter. All rights reserved.
Based on printed protocol Copyright © 1979, 1983, 1985 D. B. Fankhauser
and © 1992, 1993 J. L. Stein Carter.
Chickadee photograph Copyright © by David B. Fankhauser
This page has been accessed  times since 14 Mar 2001.
times since 14 Mar 2001.
 Robert Koch, a German physician, is famous for determining the bacteria
responsible for anthrax and tuberculosis.
The chance observation of bacteria growing on the surface of a spoiling slice
of boiled potato led Koch to realize that each spot, or colony, of bacteria
had grown as a clone from a single contaminating bacterium that had
previously landed on the potato. From this realization, Koch developed the
technique called single colony isolation, in which a sample of
bacteria from one colony may be used to inoculate a culture medium and from
that, grow a pure culture of that species of bacterium.
Robert Koch, a German physician, is famous for determining the bacteria
responsible for anthrax and tuberculosis.
The chance observation of bacteria growing on the surface of a spoiling slice
of boiled potato led Koch to realize that each spot, or colony, of bacteria
had grown as a clone from a single contaminating bacterium that had
previously landed on the potato. From this realization, Koch developed the
technique called single colony isolation, in which a sample of
bacteria from one colony may be used to inoculate a culture medium and from
that, grow a pure culture of that species of bacterium.  In his work with bacteria, Koch also developed a series of methods for determining
which species of bacterium is responsible for a given disease. In his honor,
these four steps are collectively known as Koch’s Postulates.
These say that to prove that a given bacterium causes a certain disease, the
researcher must:
In his work with bacteria, Koch also developed a series of methods for determining
which species of bacterium is responsible for a given disease. In his honor,
these four steps are collectively known as Koch’s Postulates.
These say that to prove that a given bacterium causes a certain disease, the
researcher must: