Lipids: Fats, Oils, Waxes, etc.
2D says, “Being able to store fats in our bodies is really
important to us butterflies and our caterpillars. Since our pupae are not
able to eat while all the changes that turn a caterpillar into a butterfly
are going on, the caterpillar must, first, eat enough food to store up
enough energy as body fat to last through the whole time we’re in the pupal
stage. While we Monarchs travel to Mexico to overwinter, there are other
species of butterflies that overwinter here in Cincinnati. Those butterflies
must eat enough (plant nectar) to convert lots of sugar to fat and store it
to last through the winter. Your human bodies will do the same thing if you
consume too much sugar in soft drinks. Also, while we Monarchs can get some
food along the way to Mexico, it helps out a lot if we can have a good store
of body fat built up before we leave. That both helps to insulate us during
the cooler autumn nights and gives us a bit more food to keep our wing
muscles going.” Some types of moths, including Cecropia moths, don’t even
have functional mouths as adults, and so cannot/do not eat. In those kinds
of moths, the caterpillars must eat enough and store up enough body fat to
make it through
not only their pupal stage, but also their whole adult life!
What Are Lipids?
All
Lipids
are hydrophobic: that’s the one property they have in common. This group of
molecules includes fats and oils, waxes, phospholipids, steroids (like
cholesterol), and some other related compounds.
Structure of Fatty Acids
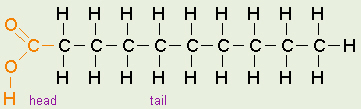
One Fatty Acid
The “tail” of a fatty acid is a long hydrocarbon chain, making it hydrophobic.
The “head” of the molecule is a carboxyl group which is hydrophilic. Fatty
acids are the main component of soap, where their tails are
soluble in oily dirt and their heads are soluble in water to emulsify
and wash away the oily dirt. However, when the head end is attached to
glycerol to form a fat, that whole molecule is hydrophobic.
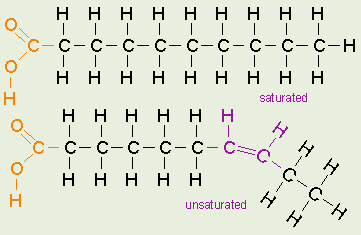
Fatty Acids
The terms saturated, mono-unsaturated, and poly-unsaturated
refer to the number of hydrogens attached to the hydrocarbon tails of the
fatty acids as compared to the number of double bonds between carbon atoms
in the tail. Fats, which are mostly from animal sources, have all single
bonds between the carbons in their fatty acid tails, thus all the carbons
are also bonded to the maximum number of hydrogens possible. Since the fatty
acids in these triglycerides contain the maximum possible amouunt of
hydrogens, these would be called saturated fats. The hydrocarbon
chains in these fatty acids are, thus, fairly straight and can pack closely
together, making these fats solid at room temperature. Oils, mostly from
plant sources, have some double bonds between some of the carbons in the
hydrocarbon tail, causing bends or “kinks” in the shape of the molecules.
Because some of the carbons share double bonds, they’re not bonded to as
many hydrogens as they could if they weren’t double bonded to each other.
Therefore these oils are called unsaturated fats. Because of the
kinks in the hydrocarbon tails, unsaturated fats can’t pack as closely
together, making them liquid at room temperature. Many people have heard
that the unsaturated fats are “healthier” than the saturated ones.
Hydrogenated vegetable oil (as in shortening and commercial peanut
butters where a solid consistency is sought) started out as “good”
unsaturated oil. However, this commercial product has had all the double
bonds artificially broken and hydrogens artificially added (in a chemistry
lab-type setting) to turn it into saturated fat that bears no resemblance to
the original oil from which it came (so it will be solid at room temperature).
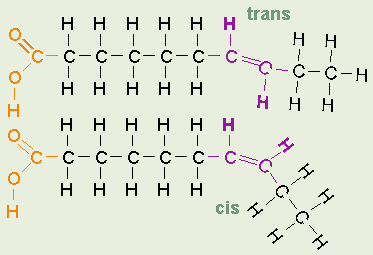
Cis and Trans Bonds
In unsaturated fatty acids, there are two ways the pieces of the hydrocarbon
tail can be arranged around a C=C double bond. In
cis bonds,
the two pieces of the carbon chain on either side of the double bond are
either both “up” or both “down,” such that both are on the same side of the
molecule. In
trans bonds,
the two pieces of the molecule are on opposite sides of the double bond, that
is, one “up” and one “down” across from each other.
Naturally-occurring unsaturated vegetable oils have almost all cis bonds, but
using oil for frying causes some of the cis bonds to convert to trans bonds.
If oil is used only once like when you fry an egg, only a few of the bonds
do this so it’s not too bad. However, if oil is constantly reused, like in
fast food French fry machines, more and more of the cis bonds are changed to
trans until significant numbers of fatty acids with trans bonds build up.
The reason this is of concern is that fatty acids with trans bonds are
carcinogenic,
or cancer-causing. The levels of trans fatty acids in highly-processed, lipid-containing products such as margarine
are quite high, and the government now requires
that the amounts of trans fatty acids in such products be listed on the labels.
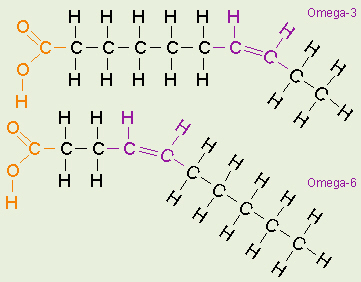
Omega-3 and Omega-6 Fatty Acids
Another set of fat-related terms that are “in the news” a lot,
lately are “omega-3” and “omega-6” fatty acids. These terms both refer to
fatty acids that have at least one unsaturated bond in their chains, and these
terms describe where that bond is located. Starting from the end of the
carbon chain (that does not contain the carboxyl group and is not bonded on
to the glycerol), the carbon atoms before the first double bond are counted.
If there are three carbons, it is an omega-3 fatty acid, and if there are
are six carbons, it is an omega-6 fatty acid. We need a balanced amount of
both in our diets, but the omega 3 fatty acids are not as common, thus harder
to obtain, and so many people’s intake of these two types of fatty acids is
way out of balance, including way too many omega-6 fats as compared to the
amount of omega-3 fats in their diets. Flax seed and chia seed contain
significant amounts of omega-3 fatty acids. Certain types of marine algae
manufacture lots of omega-3 fatty acids, and those are incorporated into the
tissues of fish which eat those algae. Because of that, many people take
fish-oil capsules to increase the amount of omega-3 fats in their diets, but
especially for vegetarians, consuming algae-derived omega-3 fats is another
option.
Structure of Fats and Oils
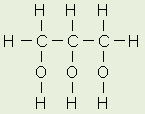
Glycerol
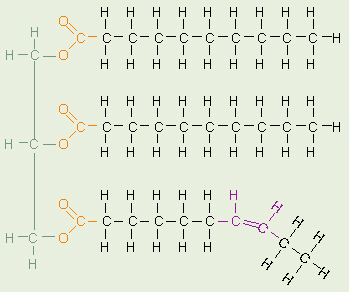
Triglyceride
Fats and oils are made from two kinds of
molecules: glycerol (a type of alcohol with a hydroxyl group on each
of its three carbons) and three fatty acids joined by dehydration
synthesis. Since there are three fatty acids attached, these are known as
triglycerides.
“Bread” and pastries from a “bread factory” often contain mono- and
diglycerides as “dough conditioners.” Can you figure out what these
molecules would look like? The main distinction between fats and oils is
whether they’re solid or liquid at room temperature, and this, as we’ll soon
see, is based on differences in the structures of the fatty acids they
contain.
The fatty acids that make up the fats and oils in our diets
and our bodies may also be lumped into groups based upon the number of
carbon atoms in their chains. Interestingly, most of the fatty acids in
living organisms have an even number of carbons (2, 4, 6,...). Fatty acids
with less than 6 carbons in their chains may collectively be called
short-chain fatty acids, but
usually these are just called carboxylic acids and are not referred to as
“fatty” acids. Those with 6 to 12 carbons are
medium-chain fatty acids, those
with 14 to 22 carbons are long-chain fatty
acids, and those with over 22 carbons are the
very-long-chain fatty acids. Since, as mentioned above, fats and oils
contain three fatty acids and are called triglycerides, those which contain
primarily medium-chain fatty acids are referred to as
medium-chain triglycerides (MCTs),
while those which contain primarily long-chain fatty
acids are referred to as long-chain
triglycerides (LCTs).
Most of the fats and oils in our diets and that we’re used to hearing about
in the news are LCTs.
| Chain Length |
# of Carbons |
Name |
Formula |
Notes |
| The first 11 saturated fatty acids are: |
|---|
| short-chain |
C2 |
acetic acid |
CH3COOH |
aceto = vinegar |
| C4 |
butryic acid |
CH3(CH2)2COOH |
butyr = butter |
| medium-chain |
C6 |
caproic acid |
CH3(CH2)4COOH |
capri = goat |
| C8 |
caprylic acid |
CH3(CH2)6COOH |
|
| C10 |
capric acid |
CH3(CH2)8COOH |
|
| C12 |
lauric acid |
CH3(CH2)10COOH |
lauri = laurel |
| long-chain |
C14 |
myristic acid |
CH3(CH2)12COOH |
myrist = anoint, ointment |
| C16 |
palmitic |
CH3(CH2)14COOH |
|
| C18 |
stearic acid |
CH3(CH2)16COOH |
stear = fat, suet, tallow |
| C20 |
arachidic or eicosanoic acid |
CH3(CH2)18COOH |
arachis = a leguminous plant, present in peanut oil |
| C22 |
behenic or docosanoic acid |
CH3(CH2)20COOH |
present peanut & canola oils |
| A few of the more important
unsaturated fatty acids include: |
|---|
EFA
= essential fatty acid, PUFA = polyunsaturated fatty acid, MUFA =
monounsaturated fatty acid.
(CH2)7, for example, means repeat CH2
seven times in a row, so CH2CH2CH2CH2CH2CH2CH2.
To determine the omega number, count the number of carbons in from the left
end of each of
the following molecules until the first double bond. Do
not start counting from the carboxyl group. |
| |
C18 |
oleic acid |
CH3(CH2)7CH=CH(CH2)7COOH |
omega-9, MUFA, present in olive & sesame oils, oleo = olive, olive oil |
| C18 |
linoleic acid |
CH3(CH2)4CH=CHCH2CH=CH(CH2)7COOH |
EFA, omega-6, PUFA |
| C18 |
linolenic or alpha linolenic acid (ALA) |
CH3CH2CH=CHCH2CH=CHCH2CH=CH(CH2)7COOH |
EFA, omega-3, PUFA, present in flaxseed oil |
| C20 |
arachidonic acid (ARA) |
CH3(CH2)4(CH=CHCH2)4(CH2)2COOH |
EFA, omega-6, PUFA |
| C20 |
eicosapentaenoic or timnodonic acid (EPA) |
CH3(CH2CH=CH)5(CH2)3COOH |
omega-3, PUFA, present in algae/fish oil |
| C22 |
docosahexaenoic or cervonic acid (DHA) |
CH3(CH2CH=CH)6(CH2)2COOH |
omega-3, PUFA, present in algae/fish oil |
Notice that, while these unsaturated fatty acids contain the same number of
carbons as some of the saturated fatty acids, they are chemically different
and have different names.
The medium-chain triglycerides (MCTs) play some very
interesting roles in our bodies.
MCTs are handled quite differently by our bodies. Our digestive tract
doesn’t need to digest them or even use bile to emulsify them, but they are
directly absorbed into our blood
and sent to the liver where some of them are converted into ketones. Both
the MCTs themselves and the ketones formed from them are directly used by
our brains and our muscles as alternate fuel/energy sources in place of
glucose. In general, MCTs are not stored as body fat, so unless a person
would consume way more than necessary, they do not contribute to weight gain.
However, consumption of “too much” is unlikely because eating more at
once than one’s body is used to tends to cause diarrhea.
Even though they are saturated fats, they are “heart-healthy,” and the
heart actually beats more effeciently using them as fuel in place of glucose.
Think what life must have been like back in
“cave-man days” — people back then weren’t assured of three sumptous,
regularly-scheduled meals a day, but rather, if they hadn’t killed a buffalo
or a gazelle in several days, they might have nothing to eat for a couple
days. If glucose (sugar) was the only fuel their brains and muscles could
use to keep going, they would have, long-ago, starved to death, and we
wouldn’t be here today. Rather, being able to use MCTs as an energy source
enabled them to keep going and survive. Even today, mothers’ milk is very
high in MCTs (10 to 17% of its fat), newborns’ brains use MCTs and
their metabolic derivatives for as much as 25% of their energy requirements,
and now, every infant formula on the market these days contains MCTs.
In terms of our brains’ abilities to use glucose as a fuel,
it turns out that the insulin produced by our pancreas that helps transport
sugar into all our other body cells (so it can be used as an energy source)
cannot cross the blood-brain barrier, but rather, our brains make their own
insulin which is used to help transport glucose into the neurons (nerve
cells, brain cells) so they can use it for fuel. In terms of pancreatic
insulin, you’ve probably heard of type I and type II diabetes, in which either
the person’s pancreas isn’t making enough insulin or else the cells that
need to take the sugar out of the blood have “broken” insulin receptors, so
even if the person’s pancreas is making enough insulin, that “message” to
take sugar out of the blood never gets received by the cells. While we
tend to think about that in terms of all the sugar that’s staying in the
blood, think for a minute what that means in terms of the cells that aren’t
getting the sugar inside when they need it.
Now, instead of the rest of the body, think of all this in
terms of the brain cells. Don’t think in terms of blood or in terms of
fluid within the brain, but think in terms of the actual brain cells, the
neurons. What if a person’s neurons were insulin resistant? That would
mean that sugar couldn’t get into the neurons to be used as energy to keep
the neurons functioning. The neurons would end up, essentially, starving
to death. Does that sound far-fetched or impossible? It turns out, based
on current research, that can and does happen. Alzheimer’s is now being
referred to as type III diabetes.
It has been observed that many people who have been diagnosed with
Alzheimer’s or other similar neurological diseases were “sugar junkies” for
years before their diagnosis. While these people weren’t showing Alzheimer’s
signs and symptoms, yet, their neurons were starving, even then, and were
sending out messages “begging” for more sugar. Unfortunately, because those
neurons were unable to get all that sugar inside themselves, it didn’t do
any good, and they began to die off. When enough neurons had died, and thus,
the person’s brain had “shrunk” enough for the deficit to be noticeable,
then the person was diagnosed with Alzheimer’s or one of the other,
closely-related neurological diseases.
But wait. . . what about MCTs? Based on information
included in the book, “Alzheimer’s Disease: What If There Was a Cure?” by
Dr. Mary Newport,
a UC Med. School graduate, many people have found that feeding MCTs (found
in coconut oil, which is around 57 to 60% MCTs) to their loved ones who have
been diagnosed with Alzheimer’s (or other neurological diseases/disorders)
appears to “feed” the neurons and keep them alive, thereby slowing the
progression of the disease, and in some cases actually improving the
person’s condition somewhat. If this topic is of interest to you, then I’d
encourage you to check out
Dr. Newport’s Web site
and/or her book (link on her Web site).
MCTs are also being used to treat some types of cancer.
Some very aggressive, rapidly-growing cancers can only use glucose as an
energy source. Thus it has been found that, if people with those types of
cancer are put on a special diet that is very low in sugar, but has adequate
MCTs, the person’s body can use the MCTs for fuel, while the cancer starves.
We need fats in our bodies and in our diet. Animals in
general use fat for energy storage because fat stores 9 KCal/g of energy.
Plants, which don’t move around, can afford to store food for energy in a
less compact but more easily accessible form, so they use starch (a
carbohydrate, NOT A LIPID) for energy storage. Carbohydrates and proteins
store only 4 KCal/g of energy, so fat stores over twice as much energy/gram
as fat. By the way, this is also related to the idea behind some of the
high-carbohydrate weight loss diets. The human body burns carbohydrates and
fats for fuel in a given proportion to each other. The theory behind these
diets is that if they supply carbohydrates but not fats, then it is hoped
that the fat needed to balance with the sugar will be taken from the
dieter’s body stores. Fat is also is used in our bodies to a) cushion
vital organs like the kidneys and b) serve as insulation, especially just
beneath the skin.
Phospholipids
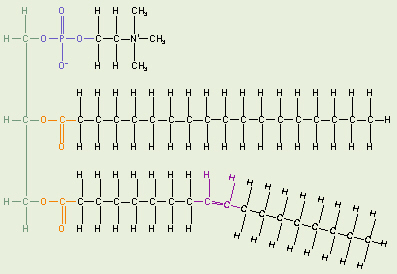
Lecithin
Phospholipids are made from glycerol, two fatty acids, and (in place of the
third fatty acid) a
phosphate group
with some other molecule attached to its other end. The hydrocarbon tails
of the fatty acids are still hydrophobic, but the phosphate group end of the
molecule is hydrophilic because of the oxygens with all of their pairs of
unshared electrons. This means that phospholipids are soluble in both water
and oil.
 An emulsifying agent is a substance which is soluble in both oil and
water, thus enabling the two to mix. A “famous” phospholipid is
lecithin
which is found in egg yolk and soybeans. Egg yolk is mostly
water but has a lot of lipids, especially cholesterol, which are needed by
the developing chick. Lecithin is used to emulsify the lipids and
hold them in the water as an emulsion. Lecithin is the basis of the
classic emulsion known as mayonnaise.
For more information on mayonnaise, see the Biol Lab 1 Mayonnaise Web page.
An emulsifying agent is a substance which is soluble in both oil and
water, thus enabling the two to mix. A “famous” phospholipid is
lecithin
which is found in egg yolk and soybeans. Egg yolk is mostly
water but has a lot of lipids, especially cholesterol, which are needed by
the developing chick. Lecithin is used to emulsify the lipids and
hold them in the water as an emulsion. Lecithin is the basis of the
classic emulsion known as mayonnaise.
For more information on mayonnaise, see the Biol Lab 1 Mayonnaise Web page.
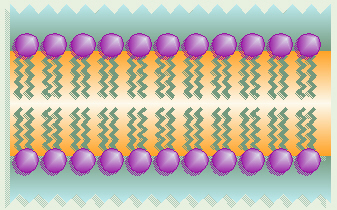
Phospholipid Bilayer
Our cell membranes are made mostly of phospholipids arranged in a
double layer with the tails from both layers “inside” (facing toward each
other) and the heads facing “out” (toward the watery environment) on both
surfaces.
Steroids
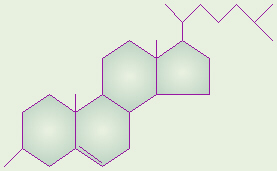
Cholesterol
The general structure of cholesterol consists of two six-membered
rings side-by-side and sharing one side in common, a third six-membered ring
off the top corner of the right ring, and a five-membered ring attached to
the right side of that. The central core of this molecule, consisting of
four fused rings, is shared by all steroids, including estrogen
(estradiol), progesterone, corticosteroids such as cortisol (cortisone),
aldosterone, testosterone, and Vitamin D.
In the various types of steroids, various other groups/molecules are attached
around the edges. Know how to draw the four rings that make up the central
structure.
Cholesterol is not a “bad guy!” Our bodies make about 2 g
of cholesterol per day, and that makes up about 85% of blood cholesterol,
while only about 15% comes from dietary sources.
Cholesterol is the precursor to our sex hormones and Vitamin D.
Vitamin D is formed by the action of UV light in sunlight on cholesterol
molecules that have “risen” to near the surface of the skin. At least one
source I read suggested that people not shower immediately after being in
the sun, but wait at least ½ hour for the new Vitamin D to be absorbed
deeper into the skin. Our cell membranes contain a lot of cholesterol (in
between the phospholipids) to help keep them “fluid” even when our cells are
exposed to cooler temperatures.
Many people have heard the claims that egg yolk contains too
much cholesterol, thus should not be eaten. An interesting study was done
at Purdue University back in 1977 to test this. Men in one group
each ate an egg a day, while men in another group were not allowed to eat
eggs. Each of these groups was further subdivided such that half the men
got “lots” of exercise while the other half were “couch potatoes.” The
results of this experiment showed no significant difference in blood
cholesterol levels between egg-eaters and non-egg-eaters while there was a
very significant difference between the men who got exercise and those who
didn’t.
Lipoproteins are clusters of proteins and lipids all
tangled up together. These act as a means of carrying lipids, including
cholesterol, around in our blood. There are two main categories of
lipoproteins distinguished by how compact/dense they are. LDL or
low density lipoprotein is the “bad guy,” being associated with
deposition of “cholesterol” on the walls of someone’s arteries. HDL
or high density lipoprotein is the “good guy,” being associated with
carrying “cholesterol”
out of the blood system, and is more dense/more compact than LDL.
References:
Borror, Donald J. 1960. Dictionary of Root Words and Combining Forms. Mayfield Publ. Co.
Campbell, Neil A., Lawrence G. Mitchell, Jane B. Reece. 1999. Biology, 5th Ed. Benjamin/Cummings Publ. Co., Inc. Menlo Park, CA. (plus earlier editions)
Campbell, Neil A., Lawrence G. Mitchell, Jane B. Reece. 1999. Biology: Concepts and Connections, 3rd Ed. Benjamin/Cummings Publ. Co., Inc. Menlo Park, CA. (plus earlier editions)
Lappé, Francis Moore. 1982. Diet for a Small Planet, 10th Anniversary Ed. Ballantine Books. New York.
Lappé, Francis Moore. 1991. Diet for a Small Planet, 20th Anniversary Ed. Ballantine Books. New York.
Marchuk, William N. 1992. A Life Science Lexicon. Wm. C. Brown Publishers, Dubuque, IA.
Newport, Mary T.
2013. Alzheimer’s Disease: What If There Was a Cure?, 2nd Ed. Basic Health Publ, Inc. Laguan Beach, CA.
Sienko, Michell J. and Robert A. Plane. 1966. Chemistry: Principles and Properties. McGraw-Hill Book Co., NY. (and other chemistry texts and handbooks)
Copyright © 1996 by J. Stein Carter. All rights reserved.
This page has been accessed  times since 15 Aug 2000.
times since 15 Aug 2000.







 An emulsifying agent is a substance which is soluble in both oil and
water, thus enabling the two to mix. A “famous” phospholipid is
An emulsifying agent is a substance which is soluble in both oil and
water, thus enabling the two to mix. A “famous” phospholipid is

