Carbohydrates
2-D says, “Yum! The flower nectar I like to drink
contains lots of sugar, and that gives me lots of energy!”
Monosaccharides
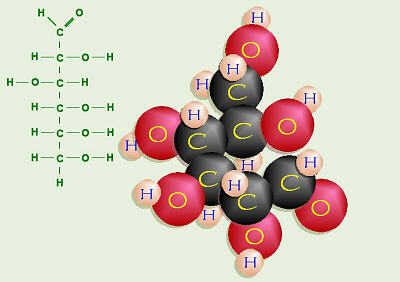
Glucose Straight Structure
Carbohydrates are defined as sugars and their derivatives. The simplest
carbohydrates are the
monosaccharides.
The most common monosaccharide is
glucose.
and this is the most important one for living organisms. Note that it
contains one carbonyl group, and since this is on an end carbon, this
makes the molecule an aldehyde. A sugar like fructose with the
carbonyl group in the middle would be a ketone (note proper spelling).
Glucose also contains five hydroxyl groups, which officially makes it an
alcohol. It is important to note on which “side” of the molecule the
hydroxyl groups may be found because the mirror image molecule is not the
same thing. Notice that all of the –OH groups except the one on the third
carbon are on the right side. These
hydroxyl groups
function differently than
hydroxide ions.
Thus, the straight structure of glucose includes a carbonyl group (–C=O) on
C-1, hydroxyl groups (–OH) on the right sides of carbons 2, 4, 5, and 6, and
a hydroxyl group on the left side of C-3. Know how to draw this.
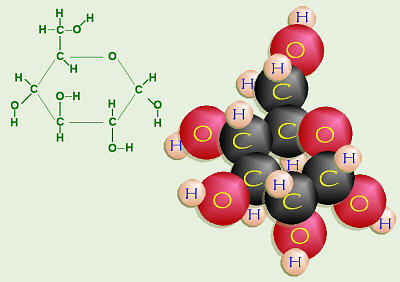
Glucose Ring Structure
In reality, what we have drawn as a straight line structure is really a
curved molecule. If you imagine C-3 and C-4 as being in the plane of the
chalkboard/paper/computer screen, then C-1 and C-2 on the top end and C-5
and C-6 on the bottom end curve around to the back. In actuality, C-1 is
very close to C-5. Because of this, glucose would rather form a ring. The
O on C-1 releases one of its bonds to C-1, and instead, bonds to the H from
the –OH on C-5. The O on C-5 then needs something else to bond to, as does
C-1 (which is no longer sharing a double bond) so the two of them bond
together, forming a ring. When the ring forms, the –OH groups on carbons 2,
4, and 5 that were on the right will be in the down positions, and the –OH
on the right of C-3, will be “up.” When the ring closes, sometimes the
“new” –OH on C-1 goes down (the α- or alpha form) and sometimes it
goes up (the β- or beta form), but both of these are still glucose.
Know how to draw this, too. To help you better visualize this, here’s
an animation showing
what the linear model really looks like in three dimensions,
and another animation showing
how
the straight form of glucose readily converts to the ring form
(note that these files are about 300 and about 400 K, so they may not be
practical to view if you have a slow connection).
Other common monosaccharides include
fructose
and
galactose.
Optical Activity of Sugars and Other Chemicals
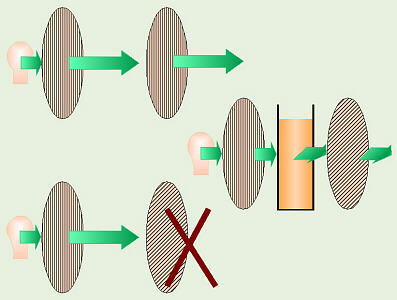
Solution Rotating Polarized Light
Many sugars have an interesting property in that they can rotate
polarized light. Normally, when light is emitted from a light source,
the light waves “vibrate” in many different directions. In polarized light,
the light waves are vibrating in only one direction. There are special
polarizing filters (remember Polaroid sunglasses?) that are used to study
this. If you shine light through one of these filters, only the light
that’s vibrating in the right direction can get through and the rest
is filtered out. If you line up a second filter going the same way, the
light can get through and the filter will look “light,” but if the second
filter is at 90°, that blocks the light, and the filter will appear
“dark.” Most sugars can rotate or change the direction the light vibrates,
so if you would place a container of sugar solution in the light path,
between the two filters, then in order to look “light,” the second filter
will have to be rotated differently to make up for how much the sugar rotates
the light. Chemists can actually identify the sugar in a solution by which
direction and how much it rotates the light. Glucose rotates polarized light
to the right so it’s also known as
dextrose.
Fructose rotates polarized light to the left, so it’s also known as
levulose.
Other sugars can rotate polarized light to the right or left, but the names
“dextrose” and “levulose” are applied only to glucose and fructose.
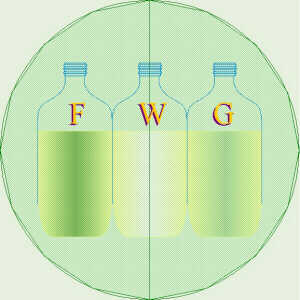
Demo of Solution Rotating Polarized Light
Try
this demonstration: As you click to the right or left, the polarizing
filters and the bottles of fructose
(F),
water
(W),
and glucose
(G)
will change color, appropriately. Watch how the bottle of fructose gets
lighter to the left and the bottle of glucose gets lighter to the right.
As a note to anyone reading this who is not one of my students here at
Clermont, this is based on the actual demo we do in class. I use autoclaved
bottles of distilled water and of 15% solutions of fructose and glucose, and
the “color” changes as the front filter is rotated are quite noticeable.
Other molecules besides sugars can also
rotate polarized light. Quite often, if there are right and left handed
mirror image forms of a particular molecule, these will each rotate polarized
light equal amounts to opposite sides. Vitamin E is one such
substance. Vitamin E actually is a group of chemicals called
tocopherols,
and the one our bodies use the most of is α-tocopherol. There
are right- and left-handed mirror images forms of this molecule, and because
of their chemical structure, one form of the molecule rotates light to the
right and the mirror-image form rotates light to the left. Therefore, these
forms are called d-α-tocopherol and l-α-tocopherol.
The d- form is natural Vitamin E, such as is made by wheat plants (present
in wheat germ). Our bodies know the difference and can only use the d- form.
If someone buys natural source Vitamin E, this only contains the d- form, and
so is all usable. However, humans are also able to make Vitamin E
synthetically in a chemistry lab, but we can’t control whether the d- or l-
form will be created, and in fact, get a 50:50 mixture of the two. Vitamin
pills that contain man-made Vitamin E, therefore contain a 50:50 mixture of
the two forms, so only half of what you’re paying for is of use to your body.
Thalidomide is a drug that was formerly given to
pregnant women to help with morning sickness. However, its use was
discontinued because of the severe birth defects it caused. Thalidomide is
also is one of those molecules with right and left mirror image forms.
Because it is a man-made drug, we can’t control whether the right vs. left
molecule forms, and again, get a mixture of the two. Someone eventually
figured out that one form was “good” in that it did help prevent morning
sickness, while the other form was the “bad” one that caused birth defects.
Because the two forms can’t be separated, this drug is no longer given to
pregnant women, although it does have some other medical uses, such as
treatment of the nausea that typically accompanies chemotherapy (to treat
cancer).
Disaccharides
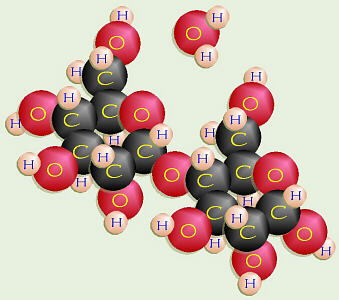
Maltose
Two monosaccharides can join by dehydration synthesis to form a
disaccharide.
For example, glucose + glucose =
maltose,
also known as malt sugar. The –OH on C-1 of one molecule and the –OH on
C-4 of another molecule collectively give up two H and one O to form water,
and are, then, joined together by the remaining O. Because this involved
C-1 and C-4, this special bond is called a 1-4-glycosidic linkage.
Similarly, glucose + fructose =
sucrose,
also known as common table, beet, or cane sugar. Sucrose rotates polarized
light to the right a little. Sometimes, in certain recipes, people dissolve
sucrose in water, then break it apart into glucose and fructose.
The mixture thus formed is called invert syrup because the fructose
in it rotates light more strongly to the left than the glucose in it does to
the right. Thus the overall net effect is that, while the original sucrose
rotated light to the right, the invert syrup solution rotates light to the
left: the rotation has been “inverted.” Honey is mostly invert syrup.
Know that if you see invert syrup listed as an ingredient on something,
it’s just another name for SUGAR!
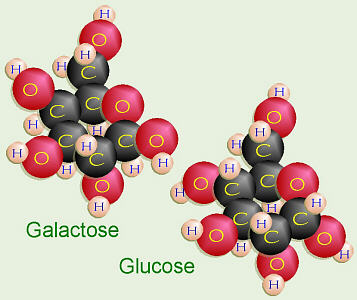
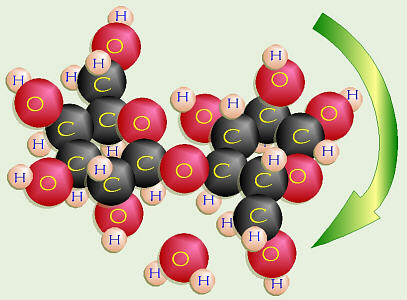
Galactose and Glucose = Lactose
Glucose + galactose =
lactose,
or milk sugar. The chemical structure of galactose is a bit different than
glucose. On C-4 of galactose, the –OH is in the “up” position. On C-1 of
galactose, the –OH is also in the “up” or β position. To make
lactose, that –OH on C-1 of the galactose has to bond to the “down” or α
–OH on C-4 of the glucose. In order to do dehydration synthesis and bond
together, the glucose has to flip over upside down so the two –OH groups
are side-by-side, thus forming a β-1-4 glycosidic linkage.
Once that bond has formed, as in milk, it takes a special enzyme,
lactase
to break this unusual bond. Some people’s bodies do not have the genetic
code needed to manufacture lactase, thus they are unable to digest the
lactose in dairy products. This undigested lactose passes through their
digestive tract until it is eventually fermented by the bacteria that
normally live in everyone’s large intestines. When this happens it often
produces “gas,” and may cause the person to have cramps and other
unpleasant symptoms. These people are called lactose intolerant
(this is different than an allergy). To help these people, synthetic
lactase is commercially available under several brand names. Also, some of
these people may be able to eat yogurt, cheese, or other dairy products in
which bacteria have already broken down the lactose.
Sweetness
Different sugars don’t all taste the same. Some taste more
or less sweet than each other. If the sweetness of sucrose, the sugar with
which most people are the most familiar, is arbitrarily assigned a sweetness
of 100%, then here’s how other common sugars compare:
| Sugar | Sweetness |
|---|
| fructose | 173% |
| sucrose | 100% |
| glucose | 74% |
| maltose | 33% |
| galactose | 33% |
| lactose | 16% |
There are several interesting things to note about this list.
There are a number of products on the market that claim to be “better for
you” because they’re sweetened with concentrated fruit juice or fructose.
Fruit juice contains all the sugar of the original pieces of fruit, and
maybe a few of the vitamins, but none of the fiber. Think how many apples’
worth of juice is in one cup of apple juice. Now, imagine that 1 C of juice
concentrated down, with most of the water removed (but none of the sugar)
until it takes up less space. That or similar from another type of fruit is
what’s used when something is fruit-juice sweetened: it’s essentially mostly
sugar. However, “they” would like you to believe that because the sugar
contained therein is mostly fructose, that it’s better for you. There are
also a number of products on the market that are specifically
fructose-sweetened. As we’ll see when we get to the discussion on cellular
respiration, one of the first steps in the process whereby your body burns
sugar for fuel is to turn glucose into fructose. Starting with fructose
just saves a step in the same chemical pathway. It turns out, then, that
most of the claims for fructose are probably based on the fact that it’s
almost twice as sweet as sucrose, therefore in theory, only half as much is
needed in a product for it to taste as sweet. Thus, the main health benefit,
if any, in fructose-sweetened products would be if they, indeed, contained
less sugar. People with diabetes or hypoglycemia (and probably everyone
else, too) should avoid these sugary products along with the rest.
Conversely, some people have been critical of dairy products containing extra
powdered milk (“non-fat milk solids”) because of the fact that lactose is so
“un-sweet” that a person could consume larger amounts without a lot of sweet
taste to warn of its presence.
Sugar Utilization by Our Bodies
Some interesting statistics on sugar consumption that I ran
across:
A little over 100 years ago, the average sugar consumption in our country was
about 40 lb./person/year. As of 1986, when Laurel Robertson, et al.
revised their book, Laurel’s Kitchen, Americans were averaging
⅓ lb. of sugar per person (including children) per day, which came to
about 127 lb./person/year, mostly from soft drinks. According to the July
1998 issue of Better Nutrition, the average American sugar consumption
then had risen to 148 lb./person/yr, the equivalent of over 600 KCal/day and
the equivalent of 30 5-lb. sacks of sugar a year or roughly 2¾ to 3 lb. per
week! Due to changes, several years ago, in labeling requirements, soft
drink manufacturers must now state on the can how many grams of sugar one
serving contains. However, since most people in our culture are not used to
thinking in grams, the listed 40 to 50 g (or more) per 12-oz. can doesn’t
really register. (Watch out for misleading labels — if you buy a soft drink
in a can, “they” say a serving is 12 oz., but if you buy the same soft drink
in a 2-L bottle, “they” say a serving is only 8 oz. That makes it look like
the soft drink in the 2-L bottle contains less sugar, but it does NOT.)
That much sugar is equal to about 3½ Tbsp of sugar per 12-oz. can. Would
you put 3½ Tbsp (about 10 tsp!) of sugar in a cup of coffee? For comparison,
4 T = ¼ C, so for every 4 to 5 cans a person drinks, (s)he is consuming 1 C(!)
of sugar. Since there are 453 g/lb, that means that consuming 10 cans of
soft drink will give a person about 1 lb. of sugar.
Also, since a 12-oz. can is equivalent to 355 mL (= 0.355 L),
an average of 45 g of sugar/0.355 L of soft drink is equivalent to 127 g/L
of sugar. A person’s blood glucose level is measured in units of mg/100 mL,
and around 80 to 120 mg glucose/100 mL (= 0.8 to 1.2 g/L) is considered
normal. (Note that 100 mg/100 mL = 1 g/L.) Normal blood volume is 80 to 85 mL
per kilogram (kg) of body weight,
so a 150-lb. person would have about 5.5 L of blood containing about 4.5 to
6.5 g of sugar, total.
Thus, one 12-oz. can of soft drink contains roughly 1/16 (0.355/5.5) of the
fluid and 9 to 10 times (~45/~5.5) as much sugar as a 150-lb person’s blood,
and the sugar in soft drinks is about 127 times (127 g/L vs 1 g/L) as
concentrated as in a person’s blood.
However, our bodies were designed to digest whole, natural
foods and work at getting sugar and other nutrients out. Humans have not
always lived with the frequent, regular, abundant food available in our
society, thus our bodies were designed to very efficiently store any
“excess” sugar beyond immediate requirements. Within reason,
naturally occurring sugars can be handled by most people, and eating whole
foods helps. For example, the fiber in whole fruit (as opposed to the lack
thereof in isolated fruit juice) helps slow down and even out the absorption
of sugar from the digestive tract into the bloodstream. Watch out for the
concentration of sugar in some “natural foods.” Apples, grapes, and bananas
are all naturally high in sugar. The diabetic exchange list allows only ½
banana at a time because they contain so much sugar. Again, apple juice
contains as much sugar as all the apples from which it was squeezed: just
think how much juice you could get out of one apple, and how many apples went
into making one cup of apple juice or cider. One raisin has all the sugar
of one grape, yet think of how many grapes a person usually consumes at one
sitting vs. how many raisins at a time.
Read packaging wisely. Manufacturers have to list ingredients
in order (largest amount, first) by how much of each substance their products
contain. Some manufacturers load their products with sugar, but then realize
that if they put sugar first on the list, maybe some wise consumers wouldn’t
purchase their products. Therefore, rather than putting in just sucrose,
they use some sucrose, some honey, some invert syrup, some corn syrup
(= dextrose/glucose), some caramel (= burnt sugar), some maltose, some fruit
juice, some fructose, etc., and then list them all separately in the
ingredients, which has the effect of moving them all down, lower on the
list. One of these products could potentially be loaded with sugar, yet
have all these things way down on the list so it looks like there’s not as
much!
Control and Effects of Blood Glucose Levels
β-islet cells in the pancreas make insulin
in response to an elevated blood sugar level. This hormone tells the liver
to take glucose out of the blood and store it as
glycogen.
If a person constantly overdoses on sugar, his/her β-islet cells may
become conditioned to constantly over-produce insulin, driving the person’s
blood sugar level too low and causing
hypoglycemia.
Eventually, the overworked β-islet cells could just give up and quit,
causing “type 2”
hyperglycemia
or
diabetes.
Conversely, the α-islet cells in the pancreas secrete
glucagon in response to a blood sugar level that’s too low. This
hormone tells the liver to turn some of the stored glycogen back into
glucose and release it to raise the blood sugar level. Also, various
hormones secreted by the
adrenal glands
also influence blood sugar level. The adrenal glands sit on top of the
kidneys, and are composed of two sections or layers: the outer cortex
and the inner medulla. The adrenal medulla, the inner portion,
secretes adrenaline or
epinephrine
and several other hormones which also tell the liver to release glucose and
raise the blood sugar level. The secretion of these hormones is triggered by
the sympathetic nervous system, which in turn, is stimulated by things like
stress, sudden fear or surprise, and/or caffeine. If someone drinks soft
drinks that contains both sugar and caffeine, several things happen. Most
obviously, the sugar in the soft drink raises the blood sugar level. If the
level goes too high too quickly, as is likely with that kind of dose of pure
sugar, the β-islet cells start producing insulin to tell the liver to
lower the sugar level. If there is too much insulin produced and the level
goes too low, this causes stress which triggers the adrenal medulla to
produce hormones to raise the level. In the mean time, the caffeine is also
triggering the adrenal medulla to produce hormones to raise the sugar level.
Again, if the level goes too high, the β-islet cells start producing
more insulin. The result of this is a blood sugar “roller coaster ride,”
where the body ends up fighting against itself.
It’s not just the blood sugar level that is affected by all
of this. While a person’s liver (and muscles) can store glycogen for future
use, his/her brain is second-to-second dependent on the blood sugar for the
energy it needs to function. Thus, I have read that if a diabetic’s blood
sugar level is too high, his/her brain has plenty of fuel, frequently
resulting in a happy, nonchalant mood and making it very difficult to
convince that person that (s)he has a problem that must be dealt with.
Conversely, a hypoglycemic, whose brain does not have enough fuel, may show
signs/symptoms of confusion, irritability or anger, drowsiness, forgetfulness,
or may even pass out. I have seen suggestions that most non-alcohol-related
(and non-cell-phone-related) traffic accidents are probably
hypoglycemia-related. Consider that many traffic accidents happen during
the 5:00 pm rush hour, that many people driving at that time may not have
eaten since lunch time, and that the last thing that many of those people
ate may have been something sugary. Often, following a “fender-bender,”
when these people get out of their cars to inspect the damage, they end up
exhibiting uncontrollable anger.
Some interesting new (as of 2012-13-14) research is showing
a link between Alzheimer’s and other degenerative nervous system disorder
and the amount of sugar available to the neurons! It turns out that insulin
from the pancreas does not cross the blood-brain barrier. Rather,
the brain makes its own insulin which enables the neurons to take sugar
inside themselves so they can use it. Many researchers now believe that
a big portion of what’s going on in Alzheimers, etc., is that, independent
of what’s happening in the rest of the body, the neurons are or become
resistant to the “message” of the brain insulin: they either are or become
insulin resistant (even though the person does NOT have either type 1 or
type 2 diabetes), to the point where Alzheimer’s is now also being
refered to as “type 3 diabetes!” This has profound effects: if the
neurons are insulin-resistant, that means they cannot get glucose inside of
themselves. If they cannot get glucose inside of themselves, they starve to
death. Interestingly, it has been observed that many people who have
Alzheimer’s were huge sugar junkies prior to their diagnosis. That makes a
lot of sense: the starving neurons send out the message that they are
“hungry” for sugar, triggering the person to crave/eat lots of sugar, BUT
that doesn’t do any good because the neurons can’t get the sugar inside of
themselves. Thus, the person keeps craving lots of sugar, but “none” of it
ever gets to the starving neurons.
This suggests a “simple” way to help “treat” Alzheimer’s and
other, similar, neurological disorders: just find an alternative fuel source
that the neurons can take in and use, thereby keeping them from “starving.”
It turns out, that may actually be a “simple,” easy thing to do!!!
Dr. Mary Newport,
a UC Med School grad, has accumulated a lot of evidence that the “unusual,”
medium-chain-triglyceride (MCT) oils in coconut oil can serve as that
badly-needed alternative fuel, helping to keep as many neurons alive for
as long as possible.
Indirectly related to sugar, but while I’m on the topic of
Alzheimer’s, etc., there’s increasing evidence (some brand-new,
recently-published research) that Alzheimer’s, Parkinson’s,
and a bunch of related disorders are linked to or caused by abnormal proteins
in the person’s brain. Many people have heard of the amyloid-β plaque
in Alzheimer’s, and there are a number of other degenerative neurological
disorders (including Alzheimer’s) that all share an abnormal protein that’s
referred to as “tau tangles” or “phosphorylated tau.” Tau starts out as a
long, straight, normal protein that’s associated with organelles called
microtubules in the neurons, and helps with their function. However, for
some reason, tau can become denatured — can lose its normal shape —
and crumple up into a tangled-up ball of protein (hence the name, tau tangles).
When this happens, those tau tangles can leave “their” neuron, go infect other
neurons, and cause their tau to tangle. The path of the infection through
the brain is often fairly evident. A similar process can happen to form
the infamous amyloid-β plaques that are characteristic of Alzheimer’s
and the α-synuclein in Parkinson’s.
These processes occur gradually, and it’s not until a significant amount
of change/death of neurons has occurred that the disease process becomes
evident: by the time a person is diagnosed with Alzheimer’s or one of the
other neurological diseases, this process has been going on for a long time,
already, and the number of dead/missing neurons has finally reached a point
where the corresponding impaired function is noticeable.
The thing is, that process of self-replication and that infective-capability
are traits that are characteristic of prions: non-living,
self-replicating, infective proteins — these were normal
human proteins that became transformed into prions and are, then, infective.
I read a research article in which the authors reported that isolated, purified,
amyloid-β plaque proteins were injected into the bloodstream of mice,
and later on, the mice’s brains were found to contain Alzheimer’s-type
changes. The authors conclude that this is indicative of the possibility of
blood-to-blood communicability of Alzheimer’s, suggesting that surgical
equipment (especially neurosurgical) that’s not treated to remove prions,
blood transfusions from an infected person, and maybe even transplacentally,
from a pregnant mother to her baby may all be possible routes of transfer.
This new knowledge sheds a bit of light on the potential
communicable/infective nature of these neurological disorders, as well as
on how they act within the brain, but also serves to point out the huge
challenge in trying to find a way to not just “treat,” but actually “cure”
these horrible diseases. The thing is, researchers are also finding out
that our brains are much more resilient than previously thought, so if only
there was a way to stop these diseases in their tracks, in many cases, the
person’s brain could at least partially heal, could make some new neurons,
and could re-learn/re-gain some of its lost function... if only...
As documented in a number of books on the subject (see list,
below), the way to deal with hypoglycemia is to eat small, high-quality
meals on a frequent basis, and AVOID SUGAR AND SIMPLE CARBOHYDRATES.
One of the very worst things a person with low blood sugar can do is to eat
candy bars, etc. in an effort to raise the blood-sugar level. What will
happen is that the level will be raised too high, too quickly, and the
β-islet cells will react by producing too much insulin. Therefore,
within 15 or 30 minutes after consumption of a candy bar, the blood-sugar
level will be as low, if not lower, and the person in worse shape than
pre-candy! If the blood-sugar level is dangerously low, a small amount
(not over ½ C) of orange juice could be consumed, but even that will have a
temporary, immediate effect, and must be quickly followed-up with some real
food. In any case, what needs to happen is that the person needs to consume
high-protein, moderate-fat, high-fiber, hard-to-digest foods that will
gradually raise the blood sugar level and keep it more constant.
By eating foods with lots of fiber and protein that are harder
to digest, and by eating these foods more often (divide daily calorie
allotment among 6 to 8 small meals), sugars from foods are put into the
system more gradually, and the person’s blood sugar level stays more constant.
Without going overboard, it is reported that fat in the diet can also help
suppress insulin secretion (good for hypoglycemics, bad for diabetics).
Any sugary foods (whether natural or man-made, such as soft drinks, cookies,
cake, candy, more than ½ C of any fruit juice, more than one apple, more than
½ banana, more than a few grapes) or foods with lots of very short chain
carbohydrates that are easily converted to sugar (white flour, white rice,
white-flour pasta, sometimes potatoes) can cause an unwanted surge of insulin,
thus should be avoided. Hypoglycemics should eat foods like whole grains
(including replacement of white bread and pasta with whole-grain bread and
pasta), legumes (peas and beans), vegetables, lower-sugar fruits like
strawberries, cheese and milk (and meat for non-vegetarians). Hypoglycemics
should always carry with them some non-perishable, high-protein foods,
such as sunflower seeds or peanuts, so that they have “emergency,”
high-protein food available at all times.
Also, just in case those sugar cravings are symptoms of early-stage neurological
problems rather than or in addition to hypoglycemia, perhaps, as Dr. Newport
suggests, the addition of coconut oil to one’s diet might be prudent.
Actually, for many hypoglycemics, just the sweet taste of
something (even artificial sweetener) may trigger the release of insulin
(remember Pavlov’s dog?), so they need to avoid even artificial sweetener,
and just have to re-train their taste buds to enjoy the taste of non-sugary
foods. Since artificial sweeteners typically are not carbohydrates and do
not contain any sugar, therefore do not raise the blood sugar level, an
insulin surge under those conditions could be even more disastrous!
From what I have read, even “normal” IV fluid can be a
problem for many hypoglycemics. Consider that, as noted above, normal
blood sugar level is about 0.1% (1 g/L), yet typical “D-5-R” IV fluid is
5% glucose, 50× as concentrated as normal blood, enough to trigger
over-production of insulin in sensitive individuals.
I have read recommendations that people with hypoglycemia should only be
given IV fluid without added glucose (plain saline, etc.), yet few hospital
staff are aware of this. A number of years ago when my husband needed some
tests that required anaesthesia, we had a terrible time trying to convince
an anaesthesiologist to use IV fluid without added sugar. He didn’t
understand that hypoglycemia = hyperinsulinism in response to sugar, and
therefore thought that dumping a bunch of sugar into a person’s system
should, somehow, alleviate the condition.
The opposite disorder is hyperglycemia = diabetes (actually
there are several types/causes of diabetes). In some forms of diabetes, the
person makes very little, if any insulin (hypoinsulinism), while other
diabetics make insulin, but the receptor sites in their livers do not
properly receive the insulin (like someone trying to call you while you have
your cell phone turned off because you’re in class — you did remember to do
that, didn’t you?). In either case, the result is that
sugar isn’t taken out of the blood for storage (the liver and muscles do
need some stored glycogen to function properly) and the blood sugar level
goes too high. Because there is very little glycogen stored in the liver
and because caffeine triggers the release of sugar from storage, I have seen
recommendations that a diabetic shouldn’t drink coffee (or other
caffeine-containing substances), since this caffeine triggers release of
stored glycogen and could deplete the supply to a dangerous level.
Polysaccharides
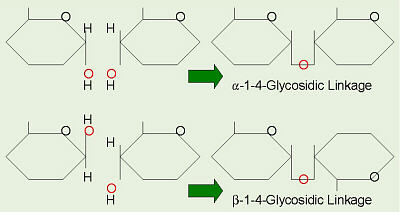
Alpha and Beta Glycosidic Linkages
Polysaccharides
are long chains of monosaccharides, like glucose molecules, all hooked
together by 1-4 glycosidic linkages formed through dehydration synthesis.
There are two main categories of polysaccharides. Storage
polysaccharides include starches and related compounds in plants, and
glycogen in animal liver and muscles. These giant molecules are made from
repeating units of glucose in the α configuration, so they can all
join together in a straight chain. These are referred to as having
α-1-4 glycosidic linkages.
Structural polysaccharides include cellulose and related
compounds. Cellulose is found in plant cell walls and is the most abundant
organic compound on Earth. This provides us with fiber in our diet, wood,
and paper. Cellulose is formed from glucose in the &beta: configuration,
and in order for the β-1-4 glycosidic linkages to form, every
other glucose molecule must flip up-side-down, as we saw in lactose. Our
bodies, and the bodies of almost all other animals, do not have the necessary
enzymes to break this β-linkage, thus we cannot digest cellulose, and
it is the fiber or bulk in our diet.
Even though we cannot digest this, it is an important part of our diet.
Without enough fiber, the digestive tract extracts too much water from the
food as it passes through, forming feces than can be very hard. This has
several consequences. These feces pass through the digestive
tract very slowly, giving various bacteria plenty of time to ferment/digest
various components, possibly releasing cancer-causing toxins into the
person’s system. The person must strain to pass these feces, causing
hemorrhoids, diverticulitis, or diverticulosis, any of which could,
potentially, require surgery. With plenty of varied fiber sources in a
person’s diet, the feces retain more water and are softer. They move
through the intestines much more quickly and are easier to pass.
Conversely, there have been people who downed several tablespoons of dry
wheat bran, then drank a glass of water. When the bran absorbed the
water and swelled, it formed a blockage that had to be surgically removed.
Be reasonable and eat a variety of whole grains,
legumes, vegetables, and fruits.
Interestingly, neither cows that eat grass nor termites that
eat wood can, by themselves, digest the cellulose contained therein.
Termites have protista that live in their guts to digest the cellulose in
the wood they eat. When a baby termite hatches out of its egg, the other
termites feed it regurgitated wood at first, and this introduces the needed
protists into its body. Cattle have a digestive system that includes a
four-chambered stomach. The first of these four chambers is called the
rumen. When a cow eats grass, the grass is first swallowed, pretty
much un-chewed, and goes to the rumen. In the rumen live special bacteria
that ferment the cellulose in the grass and produce amino acids which the
cow absorbs and uses to make protein. Eventually, the cow regurgitates the
grass and “chews its cud.” When the chewed grass is re-swallowed, it then
goes on to the rest of the stomach for further digestion. Cattle do not
need all the high-protein corn and grain we typically feed them. Because of
the bacteria living in their rumens, they can get along just fine on
grass.
References (including further information on hypoglycemia):
Abrahamson, E. M. and A. W. Pezet. 1951 (1977). Body, Mind, and Sugar. Avon Books div. Hearst Corp. New York, NY.
Borror, Donald J. 1960. Dictionary of Root Words and Combining Forms. Mayfield Publ. Co.
Campbell, Neil A., Lawrence G. Mitchell, Jane B. Reece. 1999. Biology, 5th Ed. Benjamin/Cummings Publ. Co., Inc. Menlo Park, CA. (plus earlier editions)
Campbell, Neil A., Lawrence G. Mitchell, Jane B. Reece. 1999. Biology: Concepts and Connections, 3rd Ed. Benjamin/Cummings Publ. Co., Inc. Menlo Park, CA. (plus earlier editions)
Dufty, William. 1975. Sugar Blues. Nutri-Books Corp. Denver, CO.
Fredericks, Carlton. 1985. Carlton Fredericks’ New Low Blood Sugar and You. Perigee Books div. Putnam Publ. Co. New York, NY.
Fredericks, Carlton and Herman Goodman. 1969. Low Blood Sugar and You. Nutri-Books Corp. Denver, CO.
Guest, Donna K. 1998. Test yourself: natural sweeteners. Better Nutrition. 60(7): 62.
Lappé, Francis Moore. 1982. Diet for a Small Planet, 10th Anniversary Ed. Ballantine Books. New York.
Lappé, Francis Moore. 1991. Diet for a Small Planet, 20th Anniversary Ed. Ballantine Books. New York.
Marchuk, William N. 1992. A Life Science Lexicon. Wm. C. Brown Publishers, Dubuque, IA.
Newport, Mary. 2013. Alzheimer’s Disease: What If There Was a Cure?, 2nd Ed. Basic Health Publications, Inc. Laguna Beach, CA. (and 1st Ed., 2011)
Newport, Mary. http://www.coconutketones.com/
Robertson, Laurel, Carol Flinders, and Bronwen Godfrey. 1976. Laurel’s Kitchen. Nilgiri Press. (Bantam Books Ed. 1978)
Robertson, Laurel, Carol Flinders, and Brian Ruppenthal. 1986. The New Laurel’s Kitchen. Ten Speed Press. Berkley, CA.
Sienko, Michell J. and Robert A. Plane. 1966. Chemistry: Principles and Properties. McGraw-Hill Book Co., NY. (and other chemistry texts and handbooks)
Copyright © 1996 by J. Stein Carter. All rights reserved.
This page has been accessed  times since 15 Aug 2000.
times since 15 Aug 2000.







