Cellular Respiration and Fermentation
2D says, “We Monarchs need lots of energy to live
our lives. As adult
butterflies, we siphon nectar from flowers. That is high in sugar, which
our mitochondria ‘burn for fuel,’ via the process of cellular respiration, so
our bodies can use the energy for other things, including flying all the way
to Mexico to spend the winter. While we are ‘cold-blooded,’ you might
actually see us using cellular respiration to warm up before we begin to fly.
To you humans, that might look like we’re ‘shivering,’ but by rapidly
twitching our flight muscles, thereby stimulating the mitochondria to do
cellular respiration, we’re able to warm up those muscles to prepare for
flight.”
Background Information:
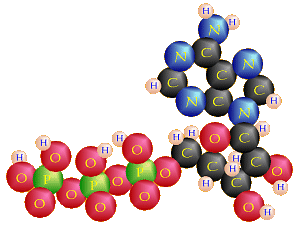
Structure of ATP
As was mentioned on the
Photosynthesis Web Page,
cells store energy as potential energy in the bonds of a chemical called
ATP (adenosine triphosphate).
Note that “ATP” is not a chemical name/formula, but is an abbreviation for
“adenosine triphosphate.”
This chemical is made of the nucleic acid adenine (also found in DNA) bonded to
ribose, a 5-carbon sugar also found in DNA, to make the nucleoside
adenosine. As in our DNA, when adenosine is bonded to a phosphate group
(-O-PO3-3, often symbolized as
℗),
that makes adenosine monophosphate or “AMP.”
Thus, ATP is very similar to the building blocks for our DNA.
AMP may be joined to another phosphate
group by dehydration synthesis to form
adenosine diphosphate or “ADP.” That, in turn, may be joined to a
third phosphate group, again via dehydration synthesis, to form ATP.
The energy may be released, again, via hydrolysis, so this chemical reaction
can go either direction, and thus may be written as
ADP + ℗ ↔ ATP + H2O
The bonds that hold
the last two phosphate groups, especially the last one, onto the molecule
are “high-energy” bonds. That means it takes a lot of energy to create the
bond, thus a lot of energy is “stored in the bond,” that bond is very
unstable, and when that bond is broken, a lot of energy will be released.
Frequently, those high-energy bonds are symbolized by a “~,” so for example,
AMP~℗~℗.
Cells use those high-energy
bonds in ATP to store the energy harvested via cellular respiration and
“transport” that energy by moving the ATP molecule to somewhere else in the
cell.
Frequently the energy is transferred to some other molecule
by transferring
the ℗ to that other molecule.
In that case, the molecule to which the ℗
is attached is said to be phosphorylated. Typically, once that molecule
has done whatever chemical reaction for which that energy was needed, the
℗ is released.
(For more information on adenine, adenosine, and AMP, see the
DNA Web Page.
For more information on dehydration synthesis, see the
Carbon Compounds Web Page.)
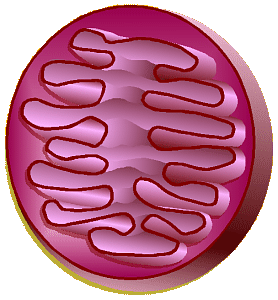
Mitochondrion
As was mentioned on the
Cells and Organelles Web Page,
Mitochondria
are found in nearly all eukaryotic cells, usually several or many per cell.
Note, by the way, that “mitochondrion” is singular and “mitochondria” is the
plural form of the word. Mitochondria are semi-autonomous: they grow and
divide on their own, they can move around within the cell and change shape,
and they contain their own DNA and thus are able to direct some of their own
protein synthesis independent of the cell nucleus (although they do
depend on other molecules which are provided by the cell). Consequently, it
is thought that mitochondria (and chloroplasts) may have originally arisen
from a prokaryote that “invaded” and “parasitized” a host cell.
Muscle and brain (nerve) cells that need a lot of energy to
do their jobs
contain an especially high number of mitochondria. That’s because
mitochondria are the “engines” of the cell: they burn sugar for fuel in the
process of cellular respiration and harvest the energy from the sugar,
transferring it to ATP so the cell can use that energy for other
purposes.
Mitochondria consist of a smooth outer
membrane and a convoluted inner membrane separated by an
intermembrane space. The convolutions of the inner membrane are called
cristae
and the space inside the inner membrane is the mitochondrial matrix.
The chemicals which make up the electron transport chain (described
below) are built into the inner membrane. As sugar is burned for fuel, a
mitochondrion shunts electrons, hydrogen ions, and a few other chemicals
across the inner membrane (matrix to/from intermembrane space).
Not directly related to cellular respiration, but there
have been some very
interesting studies done on mitochondrial DNA (mt-DNA) in humans. In humans (and other
mammals), when a sperm fertilizes an egg to create a new baby, the only
thing from the sperm cell that enters the egg cell is the sperm’s chromosomes.
None of the sperm’s mitochondria (or other organelles) enter the egg. Thus,
while we get half of the DNA/chromosomes in the nucleus of each of our cells
from our mother and half from our father, we get 100% of our mitochondria,
and therefore mt-DNA, from our mother. Studying and comparing mt-DNA has
enabled researchers to construct a “giant family tree” for humans, as well as
things like being able to tell you whether your maternal ancestors came from
Ireland or Germany or were Native American, etc.
As cells do cellular respiration, they need a way to store and transport
electrons. There are two main chemicals that are used to do this. One is
an ion called
nicotinamide adenine dinucleotide
or NAD+. The other is
flavin adenine dinucleotide
or FAD (which is not an ion).
NAD+ can combine with the proton from one and the electrons from
two hydrogen atoms, leaving behind the “other” hydrogen proton as an ion.
These hydrogen atoms (remember a hydrogen atom = 1 proton + 1 electron) come
from the chemical reactions that are taking place during cellular respiration
(described below). FAD can combine
with two hydrogen protons and two electrons (so two “whole” hydrogen atoms).
The chemical equations for these reactions are:
NAD+ + 2 H+ + 2 e– → NADH + H+
FAD + 2 H+ + 2 e– → FADH2
In many of the chemical reactions that are part of cellular
respiration,
the molecules involved are gaining or losing electrons: either receiving
electrons from another molecule, or giving electrons to another molecule.
When a substance loses electrons, we say is is oxidized, and when a
substance gains electrons, it is reduced. When I was in
school, I struggled to try to remember which is which, until one of my
chemistry professors shared with our class a really easy way to
remember which is which:
LEO the lion says GER
where “LEO” stands for “loss of electrons is oxidation” and “GER” stands for
“gain of electrons is reduction.” Typically, in this type of chemical
reaction, both reduction and oxidation will happen simultaneously because
one molecule is losing electrons as it gives them to another molecule which,
therefore, is gaining them. Because of that, chemists and biologists call
these kinds of reactions redox reactions (“red-” from “reduction” and
“ox” from “oxidation”).
Actually, you have probably heard these words used in your
daily life: for
example, “Vitamin C is good for you because it is an antioxidant.” What
that means is that vitamin C, itself, is very easily oxidized (LEO). Thus,
when “free radicals” are cruising around in your cells looking for electrons
to grab (GER), vitamin C will gladly give up some of its electrons. That’s
a “good” thing because it prevents other molecules that you need from losing
electrons, from being oxidized. That’s why vitamin C is called an
“antioxidant.” It is also called a “reducing agent” because when it gives
up its electrons (LEO), it thereby causes some other molecule to be reduced
(GER).
We will see numerous examples of redox reactions, below: for example:
4 H+ + 4 e– + 2 NAD+ → 2 NADH + 2 H+
O2 + 4 H+ + 4 e– → 2 H2O
Glycolysis:
There are two important ways a cell can harvest energy from food:
fermentation
and
cellular respiration,
also known as aerobic respiration because oxygen is used as a “final
electron acceptor.” Technically, a third way would be anaerobic respiration,
in which a cell (mostly only in some kinds of bacteria) harvests energy from
food by using the same chemical pathways as in aerobic respiration, except
that some other chemical, such as sulfur, is used as the final electron
acceptor. Typically, the kinds of bacteria that use anaerobic respiration
live in extremely-low-oxygen environments such as deep in soil or deep
underwater, and not only do they not use oxygen, but they are actually
poisoned/killed if exposed to it.
Both cellular respiration and fermentation start with the same
first step:
the process of
glycolysis
which is the breakdown or splitting of glucose (6 carbons) into two 3-carbon
molecules called
pyruvic acid.
The energy from other sugars, such as fructose, is also harvested using this
process.
Glycolysis is probably the oldest known way of producing
ATP.
There is evidence that the process of glycolysis predates both the existence
of oxygen (O2) in the Earth’s atmosphere and organelles in cells.
The Earth’s early atmosphere, in which living organisms first evolved, did
not contain O2. Those first organisms obtained the energy they
needed via fermentation or anaerobic respiration. Eventually, organisms
evolved (predecessors to plants) that were able to harvest energy from
sunlight via photosynthesis, but that process generated a toxic waste
product: oxygen! As those organisms became more numerous and released “toxic”
oxygen into the atmosphere, the oxygen level gradually increased, and
somewhere along the line, some other organisms evolved that were not
killed by the oxygen, and were also able to use it to their advantage by
being able to do cellular respiration. Consider that:
- Glycolysis does not need oxygen as part of any of its chemical reactions. It
serves as a first step in a variety of both aerobic and anaerobic
energy-harvesting reactions.
- Glycolysis happens in the cytoplasm of cells, not in some specialized
organelle.
- Glycolysis is the one metabolic pathway found in all living organisms.
Glycolysis is a process that includes a number of
steps/chemical reactions,
each of which needs special enzymes to make it happen. In the third step
in the process, glucose is converted to fructose. Thus, any fructose from
the organism’s food can be “plugged into” the process at that point.
Similarly, there are ways to chemically react other dietary sugars, and even
proteins and lipids, such that their energy can be harvested. However, keep
in mind that not all nutrients are used as “fuel” — many are used, instead,
as building blocks. What is shown below are net totals. For ATP, for
example, two of the early steps actually use 2 ATPs, while two of
the later steps make a total of 4 ATPs, and thus the net total is 2 ATPs.
None of the steps in glycolysis use or need oxygen, so as mentioned, it can
be the first stage in either aerobic or anaerobic processes.
To summarize, overall, in the process of glycolysis:
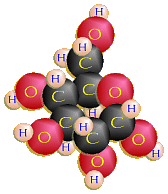
Glucose (C6H12O6)
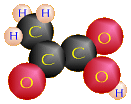

2 Pyruvic Acid (written as either C3H4O3 or CH3COCOOH) molecules
+ 
4 H+ + 4 e–
and then 2 of those electrons and one hydrogen ion combine with a NAD+
molecule to form NADH, leaving behind a hydrogen ion (H+), × 2 for
the whole glucose molecule, so

+ 

+ 
4 H+ + 4 e– + 2 NAD+ → 2 NADH + 2 H+
+ energy which is stored by making a net total of 2 ATP molecules, so:
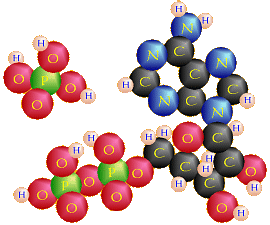

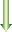


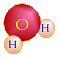

(2 ADP + 2 ℗ → 2 ATP + 2 H2O)
So, overall:
C6H12O6 + 2 NAD+ + 2 AMP-PO3H2 + 2 H3PO4→
2 C3H4O3 + 2 NADH + 2 H+ + 2 AMP-PO3H-PO3H2 + 2 H2O
(Note that, to make it easier to see
what’s going on here, these molecules have all been shown with all of their
hydrogens attached. However, for those of you who have had some chemistry,
technically, in “real life,” just like HCl is really H+ +
Cl–, so also some of the hydrogen protons on the pyruvic acid,
the lactic acid (below), and the ATP are really detached as H+
ions, leaving their electrons behind. If you haven’t had chemistry, don’t
get stressed out over this technicality.)
From there, the cell has to “do something” with the pyruvic
acid and with
the NADH that was formed. As mentioned above, that is accomplished via either
fermentation or cellular respiration. Note, by the way, that cellular
respiration is not “breathing,” but is a process whereby sugar is burned for
fuel. Coincidentally, in humans, the oxygen needed for cellular respiration
is inhaled via breathing before being distributed throughout the body via the
blood. For many other organisms, however, other means of obtaining and
distributing oxygen are used.
Fermentation:
In fermentation the pyruvic acid molecules are turned into some
“waste product,” and no more energy is produced. Note that a net
total of only two ATP molecules per molecule of glucose are produced in
glycolysis, and no more is produced during fermentation. Out of many
possible types of fermentation processes, two of the most common types are
lactic acid fermentation and alcohol fermentation (other
types of fermentation such as methanol fermentation and acetone fermentation
also exist). The various fermentation processes are all anaerobic — none
of them use oxygen.
| In lactic-acid fermentation: |
|
In alcohol fermentation: |
 + +

Pyruvic Acid (C3H4O3) + H+ + NADH |
 |
|
 |
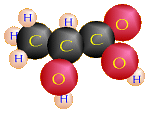

Lactic Acid (C3H6O3) + NAD+ |
or |
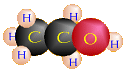


Ethanol (C2H5OH) +
Carbon Dioxide (CO2) + NAD+ |
Notice that these values are per pyruvic acid, so since there
are two molecules of pyruvic acid formed from each glucose, these numbers
would be doubled per glucose. Notice that no more ATP is formed, so the
various fermentation processes are only able to harvest 2 ATPs’-worth of
energy from a glucose molecule (as compared with 30-ish ATPs’-worth harvested
via cellular respiration, as described below).
Lactic acid fermentation is done by some fungi, some bacteria like the
Lactobacillus acidophilus.
in yogurt, and sometimes by our muscles. Normally our muscles do cellular
respiration like the rest of our bodies, using O2 supplied via
our lungs and blood. However, under greater exertion when the oxygen
supplied by the lungs and blood system can’t get there fast enough to keep up
with the muscles’ needs, our muscles can switch over and do lactic acid
fermentation. In the process of
lactic acid fermentation, the 3-carbon pyruvic acid molecules are turned into
lactic acid.
It is the presence of lactic acid in yogurt that gives it its sour taste,
lactic acid produced by the bacteria and/or fungi in many cheeses that gives
those cheeses their characteristic flavors, and
it is the presence of lactic acid in our muscles “the morning after” that
makes them so sore. Once our muscles form lactic acid, they can’t do anything
else with it, so until it is gradually washed away by the blood stream and
carried to the liver (which is able to deal with it), our over-exerted
muscles feel stiff and achy even if they haven’t been physically injured.
Note that, as mentioned, it is the hemoglobin in our red
blood cells (RBCs)
that carries oxygen from the lungs to all the cells in the body so they can
do cellular respiration (as described below). However, if someone is anemic
(technically, there are several different kinds of anemia), that typically
means that person doesn’t have enough RBCs/hemoglobin to carry enough oxygen
to the cells so they can perform as much cellular respiration as is needed to
harvest all the energy they require. In that case, the muscles will more
frequently rely on lactic acid fermentation to obtain at least a little bit
of energy to keep moving. Thus an anemic person may be more likely to have
stiff, achy muscles. This can be a concern for people with cancer if either
their cancer and/or their chemotherapy has supressed production of RBCs.
There are also some rare genetic disorders in which a person’s body lacks
one of the enzymes needed to do cellular respiration, and those people’s
muscles will also depend on lactic acid fermentation to obtain energy.
A related bit of information: while most textbooks focus on
the role of
cellular respiration in our muscles’ ability to contract and their ability to
use lactic acid fermentation when they’re short on oxygen, little mention is
made of the role of cellular respiration in our other cells’ ability to “do
their jobs” properly. One example of another very important use for cellular
respiration is that our nervous system’s ability to function properly
depends on cellular respiration. All of the nerve cells (neurons) in our
brain and the rest of our nervous system depend on cellular respiration in
their mitochondria to provide them with the energy they need to think and to
process other incoming and outgoing sensory messages. One extremely important
difference between nerve cells and muscle cells is that nerve cells cannot
switch to lactic acid fermentation if oxygen is low, but rather, our nervous
system is minute-to-minute, second-to-second, totally dependent on the oxygen
delivered by the blood. That means that if someone is anemic, therefore
lacking the hemoglobin needed to transport oxygen, therefore lacking proper
oxygen levels in his/her brain, his/her brain function will be
impaired. That person will think more slowly and perhaps be unable to follow
conversation occuring at a normal speed. Also, his/her reaction time will be
slowed, possibly to the point where it might be dangerous to drive. That
person may seem to do everything in “slow motion,” and take a long time
between each step in a task to “think about it” and process information before
(s)he is able to go on to the next step: for example, opening the refrigerator
door, then just standing there, “staring at it” for a couple minutes before
being able to remove the desired item(s). (Also note that while muscles can
also store some of the sugar they need for fuel, the brain cannot store that,
either, and so is also totally dependent on the sugar delivered by the blood.
Thus, someone who is both anemic and hypoglycemic could, potentially, have
significant problems with brain function.)
The muscles of diving mammals such as whales, porpoises, and
seals make an
interesting use of lactic acid fermentation. Their bodies have several
adaptations to allow them to hold their breath for a long time while they’re
underwater. They are able to concentrate their blood
in the center of their bodies, and because their blood is in a smaller area
of their bodies, their heart rates can be slower and their hearts do not have
to work as hard. They have more of a special oxygen-storage molecule called
myoglobin
in their muscles, enabling them to store a lot of O2 before a dive.
So that the gases in their blood don’t come out of solution during a dive and
so that lungs full of air don’t make them more bouyant, many of these diving
animals exhale before a dive and depend on circulation and metabolism
to provide the needed oxygen. While submerged, diving animals use lactic
acid fermentation to harvest the energy needed to swim, and then once they
surface and breathe in O2, they are able to re-convert the lactic
acid that has built up in their muscles back into pyruvic acid, which is then
sent through the rest of the cellular respiration process to harvest more
energy and form more ATP.
Alcohol fermentation is done by yeast and some kinds of bacteria. The
“waste” products of this process are
ethanol
and carbon dioxide (CO2). Humans have long taken advantage of
this process in making bread, beer, and wine. In bread making, it
is the CO2 which forms and is trapped between the gluten (a long
protein in wheat, rye, and barley) molecules that causes the bread to rise,
and the ethanol (often abbreviated as EtOH – do you remember how to draw it?)
evaporating that gives bread its wonderful smell while baking.
On a somewhat-related note, more and more people, these days,
are finding out
that they are gluten-intolerant, and thus, cannot eat wheat, barley, or rye.
Since it is the gluten in “regular” bread that holds in the CO2,
allowing bread to rise, manufacturers of (expensive) gluten-free bread have
experimented with using other, perhaps questionable health-wise, additives
to trap the CO2. Also, unfortunately, those manufactured breads
often also have relatively large amounts of simple starches and sugars added,
making them unsuitable for people who have hypoglycemia or diabetes, or who
are avoiding consumption of simple carbohydrates for other health reasons.
The adverse effects of the ethanol in beer and wine on the
human nervous
system are something with which many college students are familiar (sometimes
too familiar?), and it is the CO2 produced by the process of
fermentation that makes these beverages effervescent. Interestingly, since
beer and bread contain pretty-much the same ingredients: yeast, sugar, and
grains, in the past, beer has been referred to as “liquid bread.” However,
“healthy” whole-grain bread is considerably higher in protein and lower in
alcohol than beer!
| Dr. Fankhauser has a number of fermentation-related recipes
online, complete with photographs: |
 |
|
 |
Cellular Respiration:
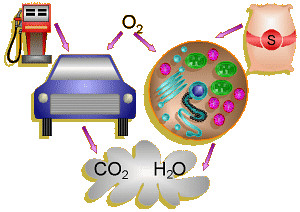
Comparison of Cell and Car
An analogy can be drawn between the process of cellular respiration in our
cells and a car. The mitochondria are the engines of our cells where sugar
is burned for fuel and the exhaust is CO2 and H2O.
Note that in a car that burned fuel perfectly, the only exhaust should
theoretically be CO2 and H2O also.
There are three steps in the process of
cellular respiration: a) glycolysis (as described above), b) the
Krebs cycle,
named after Hans Krebs who figured it out and also known as the
citric acid cycle,
and c) the
electron transport chain.
In contrast to fermentation, in the process of cellular respiration,
the pyruvic acid molecules are broken down completely to CO2 and
a lot more energy is released.
In eukaryotic cells that contain mitochondria, the Krebs
cycle and
electron transport chain occur within the mitochondria. Thus, for the
process of cellular respiration to continue, the pyruvate, NADH, and
H+ formed in glycolysis must enter a mitochondrion. Because NADH
cannot cross the mitochondrial membranes by itself, the cell must use active
transport to get NADH into the mitochondria, and that “costs” the cell one
ATP’s-worth of energy per NADH. Thus, since 2 molecules of NADH were formed
during glycolysis, that means 2 of the 4 ATPs formed during glycolysis
must give up their energy by being turned back into ADP. Prokaryotes
such as bacteria, do not contain mitochondria, and the chemicals needed to
do cellular respiration are built into other cell membranes. Because of that,
they don’t need to use energy from ATP to actively transport NADH.
In cellular respiration, there is an intermediate step in between
glycolysis and the Krebs cycle. In this step, the “end” carbon of each of
the pyruvic acid molecules (the carbon that’s
attached to two oxygens), along with those oxygens, is removed to form
a molecule of CO2, while the remaining 2-carbon molecule, called
an acetyl group, is
attached to another molecule called coenzyme A, usually referred
to as CoA. The “business end” of CoA has a sulfhydryl
group (-SH) attached to it. That hydrogen comes off and the 2-carbon
acetyl group attaches there, minus its hydrogen, to form
acetyl coenzyme A
or acetyl CoA. Those two hydrogens and an
NAD+ react to form NADH and an H+. The acetyl CoA
then carries the acetyl group into the Krebs cycle.
 +
+

Pyruvic Acid (C3H4O3) + CoA-SH
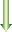
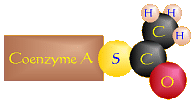 +
+
 +
+

Acetyl CoA + Carbon Dioxide (CO2) + 2 H+ + 2 e–
and then those 2 electrons and one hydrogen ion combine with
a NAD+ molecule to form NADH, leaving behind a hydrogen ion
(H+), so
 →
→ 
2 H+ + 2 e– + NAD+ → NADH + H+
Again, keep in mind that this is for “only” one pyruvate,
so for the whole glucose molecule, these numbers would be doubled.
So, overall, we now have:
C6H12O6 + 4 NAD+ + 2 AMP-PO3H2 + 2 HO-PO4H2 + 2 CoA-SH →
2 CoA-S-C2H3O + 2 CO2 + 4 NADH + 4 H+ + 2 AMP-PO3H-PO3H2 + 2 H2O
(minus 2 AMP-PO3H-PO3H2 used to transport NADH into the mitochondria of eukaryotes)
Krebs Cycle:
The acetyl group and a molecule of water are then transferred to the Krebs
cycle and bonded onto
another molecule that is part of that cycle, forming citric acid, hence the
other name for the cycle, “citric acid cycle.”
Simultaneously, the hydrogen of the sulfhydryl group on the CoA is replaced.
The CoA is
then free to go find another pyruvate with which to react. As the molecule
containing the remainder of the acetyl group goes through the eight steps of
the Krebs cycle, it gets changed, successively, into a number of different
molecules, until finally re-forming the original molecule that first picked
up the acetyl group. At two points in the process, a CO2
molecule is given off, thereby finishing the conversion of all of the
carbons in the original glucose to CO2. Also, along the way,
some NADH and ATP are formed, along with another electron carrier called
FADH2 that is similar in function to NADH. At each step in the
cycle where NADH or FADH2 is formed, since the remains of the
original molecule is losing electrons, it is, thus, being oxidized (LEO),
while the NAD+ or FAD is gaining those electrons, and thus, is
being reduced (GER).
Thus, overall, what goes into the Krebs cycle per acetyl
group (so
totals would be doubled if considered per glucose) is:
(Several molecules that enter the cycle in one
step, react, then in the next
step turn back into the same thing and leave again are not included in these
net totals.)
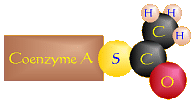







acetyl CoA (CoA-S-CH3CO) + 3 NAD+ + FAD + 2 H2O + ADP-OH + HO-PO3H2

and what comes out is:








CoA-SH + 3 NADH + 3 H+ + FADH2 + 2 CO2 + ADP-OPO3H2 (ATP)
× 2 for whole glucose =
2 CoA-S-CH3CO + 6 NAD+ + 2 FAD + 4 H2O + 2 ADP-OH + 2 HO-PO3H2 →
2 CoA-SH + 6 NADH + 6 H+ + 2 FADH2 + 4 CO2 + 2 ADP-OPO3H2
When those amounts are added to the amounts from glycolysis
and the “intermediate” step (above), the net total, so far, is:
C6H12O6 + 10 NAD+ + 2 FAD + 4 AMP-PO3H2 + 4 HO-PO4H2 + 4 H2O →
6 CO2 + 10 NADH + 10 H+ + 2 FADH2 + 4 AMP-PO3H-PO3H2 + 2 H2O
Eliminating “extra” waters that occur on both sides of the
equation, this becomes:
C6H12O6 + 10 NAD+ + 2 FAD + 4 AMP-PO3H2 + 4 HO-PO4H2 + 2 H2O →
6 CO2 + 10 NADH + 10 H+ + 2 FADH2 + 4 AMP-PO3H-PO3H2
(minus 2 AMP-PO3H-PO3H2 used to transport NADH into the mitochondria of eukaryotes)
Electron Transport Chain:

(Double-) Click on the this porphyrin ring to see how to draw one.
The electron transport chain is a system of electron carriers which, in
eukaryotes, are embedded into the inner membrane of a
mitochondrion
(in prokaryotes, these are embedded into the plasma membrane).
As electrons are passed from one compound to the next in the chain, their
energy is harvested and stored by forming ATP.
Many of the compounds that make up the electron transport
chain belong to a special group of chemicals called
cytochromes.
The central structure of a cytochrome is a
porphyrin ring
like chlorophyll but with iron in the center (remember that chlorophyll has
magnesium in the center). A porphyrin with iron in the center is called a
heme group,
and these are also found in hemoglobin in our blood.
Notice from the last chemical equation, above, that as the
glucose went
through glycolysis and the Krebs cycle, a total of 10 molecules of NADH and
2 molecules of FADH2 were formed, storing a total of 24 electrons,
which will now be used.
In the electron transport chain, the NADH and FADH2 will be
oxidixed (LEO) back to NAD+ and FAD.
Each of the cytochromes and other chemicals that make up
the electron transport chain can first gain electrons (GER), then give them
up, again (LEO). The first chemical in the chain takes the electrons (GER)
from the NADH (LEO), thus re-forming NAD+. Those electrons are
then handed off (LEO) to the second chemical (GER), then to the third,
etc. Finally, the last molecule in the electron transport chain (LEO) hands
the electrons to oxygen as the final electron acceptor (GER), and that,
simultaneously deals with an equal amount of H+. Keeping in mind
that the oxygen in the air we breathe is O2, this reaction can
be written as:
O2 + 4 H+ + 4 e– → 2 H2O
so considering all 24 electrons from all the NADH and FADH2,
this could be written as:
10 NADH + 10 H+ + 2 FADH2 + 6 O2
→
10 NAD+ + 2 FAD + 12 H2O
Note that in anaerobic respiration something else is used as the final
electron acceptor. For example, there is a group of bacteria called the
purple sulfur bacteria that use sulfur in place of the oxygen.
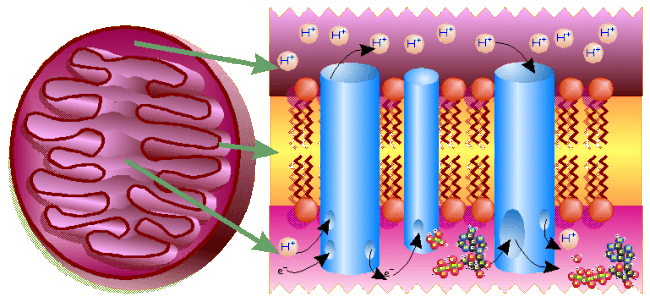
At three specific points in the electron transport chain, as
the chemical at
that point grabs an electron, it must also simultaneously grab a
hydrogen ion (H+), and then as the electron is passed on, the
H+ is also released, but there’s an interesting way in which this
must occur. At these three points in the chain, the H+ must
be taken from the mitochondrial matrix, and can only be released/given up
into the intermembrane space (as in the “left” side of the diagram, below).
Thus, the energy from the electrons is used to
pump H+ against its concentration gradient (going from lesser to
greater concentration, which it wouldn’t otherwise do) from the mitochondrial
matrix into the intermembrane space. That results in the solution in the
intermembrane space being 1 to 2 pH units lower than that in the matrix
(reminder: pH = –log[H+], so that means 10 to 100× higher
concentration of H+), and thus, has the effect of transferring
the energy from the electrons to the H+ concentration
gradient.
Because there are so many more H+ in the
intermembrane space,
they “want” to go back into the matrix to reduce the concentration gradient
and even out the concentration on both sides of the inner mitochondrial
membrane. This could/would occur via simple passive transport (diffusion)
except that the membrane is not permeable to H+. There’s only
one way they can get back in: there’s a protein built into the membrane
which serves as a passageway to allow them to re-enter. However, they
have to do a bit of “let’s make a deal” to get in. That protein that lets
the H+ back in is called ATP-synthetase or ATP-ase for short
(hopefully, seeing the “-ase” on the end of its name will give you a clue
that it’s an enzyme), and the only way the H+ can get back into
the matrix is if, at the same time, that enzyme picks up an ADP and a
℗ to make an ATP and a water
molecule (as in the “right” side of the diagram, below).
This method of making ATP, called chemiosmotic synthesis,
is different than what we saw in glycolysis and the Krebs cycle, where one
molecule just handed a
℗ to another molecule.
However, there is not a 1-to-1 chemical correspondence between number of
electrons put into the chain and the number of ATPs formed.
According to an e-mail I received from
Dr. Todd Silverstein
a chemist from Willamette University, biochemists now estimate the ratio
at about 2.3 ATPs formed per NADH, and about 1.4 ATPs formed per
FADH2. The ratio for FADH2 is lower because its
electrons are not given to the first chemical in the electron transport
chain, but enter at a later point on the chain, so not as much energy is
harvested from them.
Thus, not counting the ATPs made by the electron transport chain (since
they do not take part in the overall reaction), since we
now have the same number of NAD+ and FADH2 regenerated
and haven’t gained or lost any,
we can now change our overall reaction to:
C6H12O6 + 6 O2 + 4 AMP-PO3H2 + 4 HO-PO4H2 + 2 H2O →
6 CO2 + 12 H2O + 4 AMP-PO3H-PO3H2
but there are still 2 water molecules that can be removed from both sides, so:
C6H12O6 + 6 O2 + 4 AMP-PO3H2 + 4 HO-PO4H2 →
6 CO2 + 10 H2O + 4 AMP-PO3H-PO3H2
or
C6H12O6 + 6 O2 + 4 AMP-PO3H2 + 4 HO-PO4H2 →
6 CO2 + 6 H2O + 4 H2O + 4 AMP-PO3H-PO3H2
Thus, also “eliminating” equal numbers of atoms involved in
making ATP (in glycolysis and the Krebs cycle) and considering that as a
“separate” reaction, we
can simplify this further to the final “official” form of the overall
reaction for the “burning” of glucose via cellular respiration:
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
Given current estimates on the number of ATP molecules
produced by the
electron transport chain (as mentioned above), the overall total number
of ATP molecules produced per glucose molecule is around 28 to 30. That’s
a lot more energy harvested from one glucose molecule than the 2 ATPs that
are able to be made from the energy harvested via fermentation.
Health-Related Issues:
It is also important to note that the rates of many of the
reactions involved are controlled by feedback inhibition, which
occurs when build-up of a product
of a given step serves as an inhibitor for that (or a slightly prior)
step. One possible cause of this might be due to a genetic mutation that
causes a misshapen (nonfunctional) or missing enzyme needed to catalyze the
next reaction. Lacking that enzyme, the product of the previous step “piles
up” with “nowhere to go.” As that product builds up, its presence serves
to inhibit production of more of it, which in turn, affects the step prior
to that, etc., bringing the whole process to a grinding halt.
For example, the last cytochrome complex in the electron
transport chain is
called cytochrome oxidase (COX for short), and some people have a
genetic mutation such that they either don’t have COX or what they do have
doesn’t work. Because of that, they are unable to transfer electrons to
O2, and thus the whole electron transport chain is ineffective.
Thus, their muscles have to rely almost exclusively on lactic acid fermentation
to obtain the energy they need to move, and because their brains are so short
on energy, they typically have various neurological problems.
A number of drugs and other substances can also inhibit
specific enzymes,
thereby stopping the process. Cyanide, for example, interferes with COX’s
ability to pass electrons to O2, again, thereby stopping the whole
process.
Porphyria
is a genetic disorder in which the person’s body cannot properly
make porphyrin rings. Thus, porphyria would affect both the cytochromes in
the electron transport chain in that person’s mitochondria, causing problems
with cellular respiration, and the hemoglobin that carries oxygen in the
blood. Because there are a number of enzymes and steps involved in
forming porphyrin rings, there are a number of possible points in the process
where genetic defects could occur. The Merck Manual says there are eight
steps in the process of making porphyrin rings, with genetic abnormalities
possible in seven of the eight enzymes. My mother once had a friend who had
a type of porphyria, and this woman’s symptoms were quite variable. At times,
she would appear nearly normal while on other occasions she would have to be
hospitalized for temporary paralysis of part of her body or other symptoms.
There were a number of foods and drugs she had to avoid because they would
trigger or worsen her symptoms. She frequently was in a lot of pain.
Because the kind of porphyria she had was a dominant genetic
disorder, there was a 50% chance this woman’s daughter would also have
porphyria. Thus after the woman was diagnosed with porphyria, a number of
tests were also run on the girl, and she was more
carefully monitored as she grew up. My mother eventually lost contact with
them, so I never heard the end of the story.
Many of the enzymes in the cells of organisms need other helpers to
function. These non-protein enzyme helpers are called
cofactors
and can include substances like iron, zinc, or copper. If a cofactor is an
organic molecule, it then is called a
coenzyme.
Many of the
vitamins
needed by our bodies are used as coenzymes to help our enzymes to do their
jobs. Vitamin B1
(thiamine)
is a coenzyme used in removing CO2 from various organic compounds.
B2
(riboflavin)
is a component of
FAD (or FADH2),
one of the chemicals used to transport electrons from the Krebs cycle to the
electron transport chain. Vitamin B3
(niacin)
is a component of NAD+ (or NADH) which is the major
transporter of electrons from glycolysis and the Krebs cycle to the electron
transport chain. Without enough of these B vitamins, our ability to get the
energy out of our food would cease! B6
(pyridoxine),
B12
(cobalamin),
pantothenic acid,
folic acid,
and biotin
are all other B vitamins which serve as coenzymes at various points in
metabolizing our food. Interestingly, B12 has cobalt in it, a
mineral which we need in only very minute quantities, but whose absence can
cause symptoms of deficiency (and if overabundant, is toxic).
Some Related Topics:
Remember that plants also do cellular respiration and need O2,
too. If there is too much water
in the soil, a plant’s roots can’t get O2, and the plant “drowns”
and dies. Similarly, earthworms need the high humidity of damp soil because
they “breathe” through their skin, but they will drown in totally
water-logged soil. Thus, in a heavy rain, many earthworms come to the surface
so they can get sufficient oxygen. Unfortunately, many of them end up on our
sidewalks where they dehydrate if they can’t find a way back into the soil.
Several years ago, Dr. Fankhauser mentioned to me that he
heard that an “average” 70 kg (= 154 lb) person makes about 40 kg (= 88 lb)
of ATP/day, which would be 57% of that person’s body weight.
As we discussed that, the question arose, “What would be the hypothetical
maximum amount of ATP that a person could possibly make?”
To try to come up with a ballpark answer to that question, I did the following
calculations.
- First, let’s assume that person eats an “average” dietary intake of 2000
KCal of food energy (a number listed on the side of many food packages, but
perhaps a bit lower than a 154 lb person might consume).
- However, just out of curiosity, let’s assume that all (100%) of that is
glucose (In real life, that would be a terrible idea! We need all the other
nutrients that we get from eating a variety of foods.). Since carbohydrates
store about 4 KCal of energy per gram, that would mean that 2000 KCal of
glucose would be equivalent to 500 g (= 1.1 lb) of glucose.
Since the
molecular weight of glucose is 180 g/m, this would be equivalent to 2.78 m
of glucose.
- Also, just for the sake of argument, let’s assume that 100% of the
ingested glucose is burned for fuel, and that the process is 100% efficient
so there is no waste (in real life, our bodies would never use all 100% for
fuel – some gets used to build other chemicals, and just like the fuel
efficiency in our automobiles, the process is never 100% efficient.).
Since, as was mentioned above, eukaryotes make about 30 m of ATP from
every mole of glucose, then those 2.78 m of glucose would be equivalent
to 83.3 m of ATP.
- The
molecular weight of ATP
is 507 g/m, so that would be 42250 g or 42.25 kg of ATP.
- Thus, if it was really possible to meet all of
those background
assumptions and a 70 kg person really could make 42.25 kg of ATP, that would
be 60.36% of that person’s body weight, so that’s only a little above the
57% statistic that Dr. Fankhauser heard mentioned. Then again, there were a
lot of assumptions made that we know aren’t true.
However, to think that we make even 57% – about half – of our body weight
each day in ATP is pretty amazing.
- To look at this from another angle, suppose a
person has been severely
injured or is extremely ill with pneumonia or has had a heart attack or
stroke and is in the hospital in ICU. Typically, such a person would not
be permitted to eat anything (“NPO” in hospital lingo), and is on an IV
that is supplying various salts in concentrations that are isotonic with
the person’s blood plus 5% glucose (which is 50× more concentrated than
typical blood glucose levels), but none of the vitamins, proteins, etc.
that the person needs to fight infection and heal. At 5% glucose, each
1-L IV bag would contain 50 g of glucose, so to obtain the equivalent
of 2000 KCal of energy (500 g of glucose), would require 10 1-L IV bags,
the equivalent of 5 2-L soft-drink bottles, 2.64 gal. of fluid) per day.
Compare that with the recommended (but controversial) 8 glasses of water
we’re supposed to drink
a day (8 C = ½ gal or about one 2-L bottle). I doubt it would be common to
give someone that much fluid (10 bags/da = 1 bag/2.4 hr), so that would mean
that, in addition to having
no vitamins or protein, and in spite of the person’s pancreas having to
deal with a glucose concentration that’s 50× higher than normal blood levels,
overall the person’s system is also not getting enough energy (Calories)
to meet their needs to help them heal.
As another example:
- suppose a person would consume one 12-oz. can of soft drink,
- most types of soft drink contain about 41 to 49 g of sugar, so let’s say
this soft drink contains 45 g,
- suppose all of that sugar would be glucose,
- suppose the person’s body burns all of that sugar for fuel and does not
store any of it as fat or use any of it in other ways, and
- suppose the process of cellular respiration is 100% efficient and the
sugar is completely oxidized to CO2 and H2O.
Then:
- since the molecular weight of glucose is 180 g/m, the 45 g of glucose would
be 0.25 m,
- since cellular respiration produces about 30 m ATP for each 1 m of glucose,
that would make 7.5 m of ATP, and
- since the MW of ATP
is 507 g/m, that would be equivalent to 3802 g (about 8.38 lb) of ATP.
I once received an e-mail from a student who asked how long
the whole process of cellular respiration takes.
While I have never seen any information on that in print, a rough
approximation can also be calculated from the above statistic:
- If, as mentioned above, an “average” 70 kg person
makes about 40 kg of ATP/day, then
40 kg/24 hr × 1 hr/60 min × 1000 g/kg = about 27.8 g/min.
- Since the molecular weight of ATP
is 507 g/m, then
that 27.8 g/min × 1 m/507 g = 0.0548 m/min.
- Avagadro’s number says that there are always
6.02 × 1023 molecules/mole,
so 0.0548 m/min × 6.02 × 1023 molecules/mole =
3.30 × 1022 molecules/min.
- or, since there are 60 sec/min, then that’s
3.30 × 1022 molecules/min × 1 min/60 sec = 5.50 × 1020
molecules/sec made by a 70 kg body.
- so that would be equivalent to
5.50 × 1020 molecules/sec ÷ 70 kg = 7.85 × 1018
molecules/sec/kg of body
- or × 1 kg/1000 g = 7.85 × 1015 molecules/sec/g of body
- or × 1 g/1000 mg = 7.85 × 1012 molecules/sec/mg of body
- or × 1 mg/1000 µg = 7.85 × 109 molecules/sec/µg of body.
We can’t make computers that can do almost 8 GB/sec, yet here, we’re talking
about a tiny speck of body tissue that you’d need a microscope to see!
Check out Dr. Fankhauser’s pictures of the molecules involved in
glycolysis
and the
Krebs cycle.
References:
Berkow, Robert, ed. 1999. The Merck Manual. 17th Ed. Merck, Sharp & Dohme, Rahway, NJ.
Borror, Donald J. 1960. Dictionary of Root Words and Combining Forms. Mayfield Publ. Co.
Campbell, Neil A., Lawrence G. Mitchell, Jane B. Reece. 1999. Biology, 5th Ed. Benjamin/Cummings Publ. Co., Inc. Menlo Park, CA. (plus earlier editions)
Campbell, Neil A., Lawrence G. Mitchell, Jane B. Reece. 1999. Biology: Concepts and Connections, 3rd Ed. Benjamin/Cummings Publ. Co., Inc. Menlo Park, CA. (plus earlier editions)
Marchuk, William N. 1992. A Life Science Lexicon. Wm. C. Brown Publishers, Dubuque, IA.
Silverstein, Todd. 2010. pers. comm.


Copyright © 1996 by J. Stein Carter. All rights reserved.
This page has been accessed  times since 15 Aug 2000.
times since 15 Aug 2000.















 +
+





