Linked and Sex-Linked Genes
Linked Genes:
The dihybrid cross we previously did assumed the genes were
on different pairs of chromosomes. Now, we want to look at an example where
the genes involved are on the same chromosome. One such example is the
flower color and pollen shape experiment done by Bateson and
Punnett. In the plants that they studied, the genes for pollen shape
and flower color are located on the same chromosome (pair) as each other,
thus are inherited together.
Linked Genes
If the parents are PPLL × ppll, the first parent will only make
gametes with PL and the second with pl, which doesn’t seem too
different so far. From these parents, the F1 generation would all
be PpLl. However, when calculating what the F2 generation
will be, since the genes are located on the same pair of chromosomes, then
theoretically, the only possible gametes are PL and pl (not
Pl or pL). So
|
| PL |
pl |
| PL |
PPLL |
PpLl |
| pl |
PpLl |
ppll |
The phenotype ratio for this cross is 3:1, not 9:3:3:1 as
would be expected for a “normal” dihybrid cross. Because these genes are on
the same chromosome pair, they are called
linked genes.
Interestingly, Bateson and Punnett’s results showed just a few, unexpected
ppL- and P-ll offspring, more than would be predicted by
linked genes, but far less than would be predicted by unlinked genes in a
“regular” dihybrid cross. This is due to the fact that occasionally, during
synapsis in meiosis I, while the homologous chromosomes are paired up,
sister chromatids from the homologous chromosomes exchange equal
segments. This is called
crossing over.
In the flower example, a few of the plants could exhibit crossing over
during meiosis I, producing a few pL and Pl gametes, which
would account for the small number of ppL- and P-ll offspring.
T. H. Morgan and his grad students, who studied fruitflies, found that
the farther apart two genes are on a chromosome, the more likely there is to
be crossing over between those two genes. They found that for any given two
genes on the same chromosome as each other, the amount of crossing over that
occurs is a fairly constant quantity that can be measured. From their
crossing over data, Morgan et al. were able to arrange fruit fly
genes in the order in which they occur on the fruit fly chromosomes.
Interestingly, if two genes are very far apart on the same chromosome pair,
there is so much crossing over that the results obtained look like a regular
dihybrid cross between unlinked genes.
Linked Genes and Crossing Over in Fruit Flies
Normal fruit flies
have grayish-yellow bodies, red eyes, and wings that are long-enough to be
able to fly. As Morgan and his students were breeding fruit flies, they
found mutant flies with black bodies, some with stumpy, vestigial wings,
and some with a brighter, orangish-red eye color that they called “cinnabar,”
and by breeding flies, they were able to determine that all three of these
mutations were recessive and were on the autosomes.
Fruit fly researchers use a different type of symbolism to represent their
genetic crosses. Those researchers use a plus sign (+) to indicate anything
that is the “wild” type and a letter or two to represent a mutant allele
(capital for dominant, lower case for recessive). Thus a fruit fly with a
homozygous grayish-yellow body would be labeled as “++” while a black-bodied
fly would be “bb” and a heterozygous fly would be “+b”. The symbol for
vestigial wings is “vg” and the symbol for cinnabar eyes is ”cn”.
Through breeding, Morgan and his students were able to obtain flies that had
both a black body and vestigial wings (bbvgvg). They bred some of those
flies with wild type flies to obtain flies that were heterozygous for both
traits (+b+vg). Then they did a testcross by crossing the +b+vg heterozygous
flies with bbvgvg flies, and counted a total of 2300 offspring.
If the genes were on different chromosomes, the Punnett square for this cross
would look like this:
| |
++ |
+vg |
b+ |
bvg |
| bvg |
+b+vg |
+bvgvg |
bb+vg |
bbvgvg |
so out of the 2300 offspring, they would expect to get 575 of each of the
four types.
If the genes were linked on the same chromosome, the Punnett square for this
cross would look like this:
so out of the 2300 offspring, they would expect to get 1150 of each of the
two types.
However, when Morgan and his students actually counted the offspring, they
were surprised by the results:
| +b+vg |
+bvgvg |
bb+vg |
bbvgvg |
| 965 |
185 |
206 |
944 |
Since most of the flies were +b+vg and bbvgvg, that suggested that the two
genes were linked. However, the researchers noticed that
| (206 + 185) | × 100 = 17% |
| 2300 |
of the flies were recombinant types: they exhibited a different
combination of phenotypes than either of their parents.
Morgan suggested that this was due to an exchange of equal chromosome
segments during synapsis in meiosis which he called crossing over.
Morgan and his students subsequently determined that the gene for cinnabar
eyes was also linked to, was on the same chromosome as, black body and
vestigial wings. They determined that the rate of crossing over between b
and cn was about 9%, and between vg and cn was about 9.5%. Alfred
Sturtevant, one of Morgan’s grad students, suggested that the farther apart
genes are on a chromosome, the greater the chances of crossing over.
Thus, he suggested that the rates of crossing over (recombination frequency)
could be used to predict the order in which the genes occur. In this case,
Sturtevant said that, based on the observed recombination frequencies, cn
was in between b and vg.
Eventually, Sturtevant and his fellow students were able to arrange four
groups of fruit fly genes. Since other researchers had found four sets of
chromosomes in fruit flies, this added further evidence to support the idea
that genes are on chromosomes.
Sex-Linked Genes:
There is yet another, unrelated, special case that means
something totally different, yet has a similar-sounding name (just to
confuse freshman biology students?). This is
sex-linked genes,
genes located on one of the sex chromosomes (X or Y) but not the
other. Since, typically the X chromosome is longer, it bears a lot of genes
not found on the Y chromosome, thus most sex-linked genes are X-linked
genes. One example of a sex-linked gene is fruit fly eye color (one
of the main genes for that — there are several genes involved).
An X chromosome carrying a normal, dominant, red-eyed allele would be
symbolized by a plain X, while the recessive, mutant, white-eyed
allele would be symbolized by X' or Xw. A fly with
genotype XX' would normally be a female with red eyes, yet would be a
carrier for the white-eyed allele. Because a male typically only has
one X chromosome, he would normally be either XY and have normal, red
eyes, or X'Y and have white eyes. The only way a female with two X
chromosomes could have white eyes is if she would get an X' allele from both
parents making her X'X' genotype. The cross between a female carrier and a
red-eyed male would look like this:
Notice that while there is a “typical” ratio of ľ red-eyed to
Ľ white-eyed, all of the white-eyed flies are males.
Typically, X-linked traits show up more in males than females
because typical XY males only have one X chromosome, so if they get the
allele on their X chromosome, they show the trait. If a typical XX female
is a carrier, 50% of her sons will get that X chromosome and show the trait.
In order for an XX female to exhibit one of these X-linked traits, most of
which are recessive mutations, she would have to have two copies of the
allele (X'X'), which would mean that her mother would have to be a carrier
and her father have the trait so she could get one allele from each of
them.
In humans, two well-known X-linked traits are
hemophilia
and red-green colorblindness. Hemophilia is the failure (lack of
genetic code) to produce certain substance needed for proper
blood-clotting, so a hemophiliac’s blood doesn’t clot, and (s)he
could bleed to death from an injury that a normal person might not even notice.
One of the most famous genetic cases involving hemophilia goes back to Queen
Victoria. While both of her parents were perfectly normal, it is usually
assumed that a chance mutation in either the egg or sperm that came together
to make her, caused her to be a carrier for the hemophilia allele
(XX') [see the box, below, for an alternative hypothesis that some people
have suggested]. When she grew up, she married Prince Albert, who was
normal XY, so the Punnett square for their marriage would look like the one
just drawn. The Punnett square would predict that ˝ of their sons (Ľ
of their children) would be hemophiliacs and ˝ of their daughters (Ľ of their
children) would be carriers. Their children married other royalty, and
spread the gene throughout the royal families of Europe.
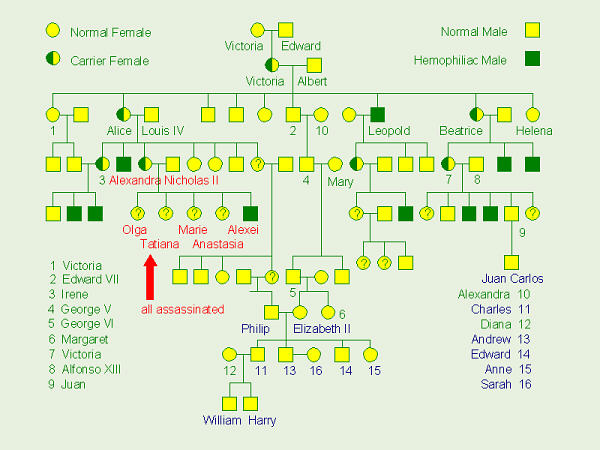
Royal Pedigree
Genetics Plays a Role in History
Queen Victoria’s
daughter, Alice, married a German prince, Louis, and converted to Lutheranism.
Their daughter, Queen Victoria’s granddaughter, Alexandra was, thus, a German
princess, grew up in Germany, and was raised in the Lutheran church.
Alexandra, married Tsar Nicholas, the last tsar of Russia, and they had four
daughters: Olga, Tatiana, Marie, and Anastasia. Many people in Russia
didn’t like Tsarina Alexandra because she was German, not Russian, and
Lutheran, not Russian Orthodox. Her mannerisms, speech, and dress were not
what many people in Russia thought of as appropriate for the Tsar’s wife.
Also, in Russia at that time, only a male could be tsar, so unless Alexandra
and Nicholas had a son, the leadership would pass to another of Nicholas’
relatives when he died. Finally, however, they had a son who they named
Alexei. Unfortunately, however, they soon discovered that he had inherited
the hemophilia allele from Alexandra, from Alice, and from Queen Victoria.
Realizing that chances were very slim that Alexei would survive to adulthood,
Tsar Nicholas and his family became very withdrawn to try to keep that a
secret (Alexandra was not very outgoing, anyway, which the people didn’t
like). However, at that time, there was much social unrest in Russia, and
the general public mistook the royal family’s withdrawl for aloofness and as
a sign that they didn’t care about the poor living conditions of their people.
Thus, Alexei’s hemophilia was probably a major contributing factor in the
Russian revolution. On several occasions, Alexei had severe internal
bleeding, and a rather disreputable man named Rasputin was somehow able to
stop the bleeding. Because of his inexplicable ability to help Alexei,
Rasputin became part of the “inner circle” and close confidant of the royal
family, which also angered many people who did not trust him.
Thus, when the Russian Revolution began, Rasputin was among the first to be
executed. Eventually, Tsar Nicholas and his family were put under house
arrest in Siberia. On 18 June 1918, Anastasia, the youngest of the daughters,
turned 17 while the family was still under house arrest, and about a month
later, just after midnight on 16 July, the royal family and several of their
servants were all ordered down into the basement of the house, and the
soldiers who had been guarding them shot and killed them all. Then, their
remains were taken out of town, burned in a bonfire, then buried, together,
in an unmarked grave. For years, no one knew where that grave was until,
when Communist rule ended, records became available. In 1991, what was
thought to, perhaps, be that grave was found, the bones were carefully
removed, and as much as possible, the skeletons were reconstructed. Through
the use of modern DNA technology, DNA samples from the bones were compared
to DNA from the Tsar’s brother’s body (buried in a crypt in a church in St.
Petersburg) and to DNA from someone in the English royal family. On that
basis, one adult male skeleton was identified as the Tsar, several young
adult female skeletons were identified as several of the daughters, and the
DNA of several of the other skeletons didn’t match, showing that they were
unrelated, family servants. The skeletons of Alexei and one of the four
daughters were not with the rest, and are still unaccounted for (I’ve
subsequently read that another grave was found nearby,and it is thought
that probably contains their bones). After the bones were studied and
identified, a few years ago, the remains of the last Tsar of Russia and his
family were given a proper funeral and burial.
In 1919, a young woman jumped off a bridge in Berlin, Germany and was rescued
and hospitalized. While in the hospital, on one occasion she showed a
magazine article with a photo of the Russian royal family to a nurse,
pointing out to the nurse how much she thought she looked like Anastasia.
After that, she claimed to be Anastasia and claimed to have escaped and
survived. She later moved to the U. S. and went by the name of Anna
Anderson. The rest of her life, she stuck to her story that she was
Anastasia, but people were dubious and tried everything they could think of
(including things like comparing pictures of ear lobes) to figure out whether
she was Anastasia, or not. When she died and was cremated in 1984, no one
still knew if she was really Anastasia or not. At some point before her
death, she had had surgery, and the hospital had kept the removed tissue
preserved in formaldehyde. Again in the 1990s, with the advent of modern
DNA technology, scientists were also able to test DNA samples from her
preserved tissue and compare those to the other DNA samples, with the
result that there were no similarities – she was not related.
Another possible use for DNA technology has been suggested. The big question
in all of this is, “From where did Victoria get the hemophilia allele?”
Neither her mother, Victoria, nor her father, King Edward showed any signs
of having that allele. The “standard” explanation which, for many years,
has been offered to freshman biology students is that there was a chance,
random mutation in that allele on one of Queen Victoria’s X chromosomes.
More recently, however, I have heard suggestions (from people that weren’t
around back then, and so don’t really know the story) that, allegedly, at that time, if
the royal couple was having trouble conceiving a child, it would not have
been out of the question to quietly, unobtrusively “loan” the Queen out.
Certain people have raised the suggestion that maybe King Edward is not
Victoria’s biological father. It has been suggested that perhaps there was
not a chance mutation in one of Queen Victoria’s X chromosomes, but that,
perhaps, that was inherited from another man. Since the bodies of deceased
members of the royal family are in crypts in Westminster Abbey, it would be
fairly easy to lift the lids on a couple of crypts to get DNA samples for
comparison, but needless to say, the British royal family probably isn’t
very enthused about that idea.
Again, colorblindness and hemophilia, while rare overall, are
more common in XY males, because they only have one X chromosome. For an XX
woman to be colorblind, for example, her mother would have to be a carrier
for the trait and her father would have to be colorblind. If by some chance,
considering the overall rareness of the allele, two such people met and
married, 50% of their daughters would be colorblind.
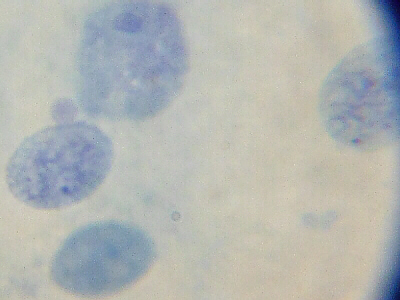
Female Buccal Cells: Barr bodies are small black dots
noticeable in cells/nuclei at 9:00 and 2:30
We have previously mentioned that it’s very important to have exactly two
copies of each chromosome (one from the mother and one from the father), and
more or less chromosomes would be an abnormal number that can cause problems.
How is it, then, that we can get by with females being XX and having two
copies of all of the genes on the X chromosome, while males, being XY, only
have one copy of most of those genes because there are no corresponding places
on the Y chromosome? Dr. Barr noticed a dark spot in the nucleus of
each cell in the body of female mammals. Mary Lyon figured out what
this was and what was going on here. In a female
mammal,
during embryonic development, one X at random is turned off in each of her
cells and condenses to form the dark spot. Mary Lyon called these inactivated
X-chromosomes
“Barr bodies”
in honor of Dr. Barr. She also figured out that as those embryonic cells
divide, all daughter cells of each of those cells will have the same X turned
off.

This is illustrated by calico cats. Coat color in cats is an
X-linked gene, with alleles for black and orange-brown, so
XBXB and XBY cats will have a black coat,
while XOXO and XOY will have an
orange-brown coat. Another possible combination for female cats would be
XBXO. Both of the color alleles would be expressed,
so the cat would end up being partially brown and partially black.
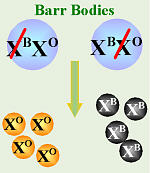
Origin of Calico Coat
As mentioned, during embryonic development, one X, at random, turns off in
each cell in a female’s body. For a cat who is XBXB
or XOXO, since both Xs are the same, this won’t be
noticed, but if a female is XBXO, in some of her cells
the XB will be turned off while in others, the XO will
be turned off. As these cells multiply by mitosis, this will lead to
patches of skin where black hair will be produced, while other patches will
produce orange-brown fur. She will end up with the patchy coat color
typical of calico or tortoiseshell cats. There is a similar, X-linked gene
in human females for the presence of sweat glands in the skin. A woman who
is heterozygous for this gene will have patchy skin containing some areas
with and some without sweat glands. This discussion will hopefully lead you
to think of several “what-if” questions:
- Q: Can a male cat ever be calico?
- A: Yes, if he’s XXY, which
would be abnormal. It turns out that abnormal numbers of X chromosomes
aren’t as serious as other chromosome number abnormalities, because all
but one X turn off, so an XXX individual would have two Barr bodies, an
XXY individual would have one, etc. (By the way, that’s an “easy” thing to
test: as in the photo, above, all that’s needed is a sample of some of the
“loose” cheek cells from
inside of someone’s mouth. Those are stained with a special dye, and if Barr
bodies are present, they will show up as a “black spot.” )
- Q: What about hemophilia? Does that exhibit a patchiness in a female
carrier (XX')?
- A: Well, sort of, but it’s not visible. The clotting
factor involved is made in the liver and secreted into and mixed with the
blood. Thus, in a carrier, some liver cells are able to manufacture the
clotting factor, while others cannot. Actually, female carriers can exhibit
a whole range of amount of clotting factor in their blood depending on how
many of which type of liver cell (X) or (X') are functioning.
But, . . . What Is “Sex,” Anyway?
We’ve been referring, here, to an organism with XX chromosomes
as “female” and with XY chromosomes as “male,” but technically, that’s not
really right. Sex is not a genotype, and it’s not right to assume
that the mere presence of XX or XY determines an organism’s sex. Rather,
sex is a phenotype that is dependent upon how a number of
genes/alleles are expressed and interact with each other. In humans, there
is a gene on the Y chromosome that codes for the presence and development of
testes, and if those testes are formed, then, under guidance from other genes,
they will begin to produce testosterone and other hormones that, in turn, are
able to stimulate development of male genitalia. (Beard quality, by the
way, is a totally separate, autosomal trait with its own genes/alleles, and
its expression/phenotype is influenced by a variety of factors.) However,
for development of male genitalia to happen, another gene, which is located
on the X chromosome and which codes for the presence and functioning of
testosterone receptors, must also do so. Interestingly, in human embryonic
development, development of female genitalia is the “default” condition, so
if there is no Y chromosome, there are no instructions to form testes and
the baby develops as a girl, but even if there are testes and testosterone,
and there’s also an alternate allele that codes for “faulty” or missing
testosterone receptors, the baby still developes as a girl. Thus, the mere
condition of being, chromosomally, XY, does not automatically mean
that person is male! Again, sex is a phenotype, not a genotype. As
described below, while it is not a very common thing, it is entirely possible
that someone could have an X and a Y chromosome, yet because of the ways in
which her alleles/genes are expressed, be phenotypically, female. In the
past, before people knew about and were able to test for X and Y chromosomes,
such a woman might have been labeled as “barren” or “infertile” – a
bad-enough label, but now that we know about X and Y chromosomes and can
test for their presence, some people, including some doctors and researchers,
forgetting that sex is a phenotype, not a genotype, much less a karyotype,
incorrectly and callously try to label these women as “chromosomally male”
– a term which is sheer nonsense.
The “opposite” condition is also possible. A colleague told
me of a case in which a couple who were having problems conceiving a baby
went to a fertility specialist, and it was discovered that the very
masculine, fully-bearded husband wasn’t producing sperm because he happened
to be XX. Also, sex determination works differently in different species of
animals. In humans and other mammals, due to the presence of Barr bodies,
the expression of the genes/alleles on the Y chromosome “normally” results
in a male phenotype, and thus people who are XXY (Klinefelter’s syndrome)
are “normally” male. In comparison, in fruit flies, genes for some sexual
traits are located on the autosomes and the ratio of the number of X and Y
chromosomes determines the sex of the fly, so while an XY fly is normally
male, an XXY fly typically is female. In grasshoppers, there is no Y
chromosome, so a grasshopper with one X chromosome (symbolized as XO) is
normally male, while a grasshopper with two X chromosomes (XX) is normally
female. In birds and butterflies, sex determination works the “opposite” of
mammals, so rather than confusing things by using X and Y to represent their
sex chromosomes, typically the letters Z and W are used. Thus a male bird
or butterfly typically has ZZ sex chromosomes, while a female typically has
ZW. In bees and ants, there are no sex chromosomes, and diploid individuals
typically show the female phenotype, while haploid individuals typically
show the male phenotype. Thus while the mode of sex determination varies
among different groups of animals (and plants), it is still true for all of
them that sex is a phenotype, and that maleness or femaleness depends on the
outcome of how that organism’s genes/alleles are expressed.
Androgen Insensitivity Syndrome (AIS) — A
Sex-Linked Gene that Helps to Determine Sex
Our genes, our genetic make-up,
is/are not independent of the rest of our bodies, but rather, are closely
integrated in and with all of our body processes. Androgen Insensitivity
Syndrome (AIS) may be used to illustrate how a person’s genetic make-up,
hormones, biochemistry, embryonic development, and phenotype are all closely
tied together and integrated, and may serve as a good example of how our
“sex” is also a phenotype that is under genetic control.
Hormones
are chemical “messengers” which are made in specific organs in our bodies,
called
endocrine glands.
These hormones travel, via the blood, to other areas of the body where they
exert chemical control over some process that is occurring in that location.
For example, the hormone insulin is made by the pancreas and travels to the
liver, where it “tells” the liver to take sugar out of the blood and store
it up by making
glycogen.
For many of our hormones, reception of their message is dependent upon
proper functioning of other chemicals in the cells of the target organs.
For example, in type II diabetes, the person’s body is making adequate
insulin, but the insulin receptors in his/her liver are not
functioning properly, so the liver never gets the message to store up sugar,
and the person’s blood sugar level goes too high. (As an analogy, it’s
similar to a situation in which someone might be trying to call you on your
cell phone, which you remembered to turned off before going to class, so
the “receptor” is not working so you don’t get the call.)
 Androgens,
including testosterone, are hormones which all of us, both men and women, make in our bodies. In both men and women, testosterone is responsible for the coarse pubic and axillary (armpit) hair
which starts to grow at puberty. Since the testes are the primary organs which produce testosterone, people with testes typically have a higher level of
testosterone in their bodies than people without testes, and that is responsible for development of most of the traits that we consider “male”. As in
the above example, the testosterone produced by the testes is secreted into the blood and travels to many other areas of the person’s body to exert its effects, and also as above, testosterone (androgen)
receptors are required in those target locations. By the way, all of us, both men and women, also make at least some estrogen, and for all of us, both men and women, how we look – our phenotype
– is typically influenced by the effects of both the testosterone and estrogen in our bodies.
Androgens,
including testosterone, are hormones which all of us, both men and women, make in our bodies. In both men and women, testosterone is responsible for the coarse pubic and axillary (armpit) hair
which starts to grow at puberty. Since the testes are the primary organs which produce testosterone, people with testes typically have a higher level of
testosterone in their bodies than people without testes, and that is responsible for development of most of the traits that we consider “male”. As in
the above example, the testosterone produced by the testes is secreted into the blood and travels to many other areas of the person’s body to exert its effects, and also as above, testosterone (androgen)
receptors are required in those target locations. By the way, all of us, both men and women, also make at least some estrogen, and for all of us, both men and women, how we look – our phenotype
– is typically influenced by the effects of both the testosterone and estrogen in our bodies.
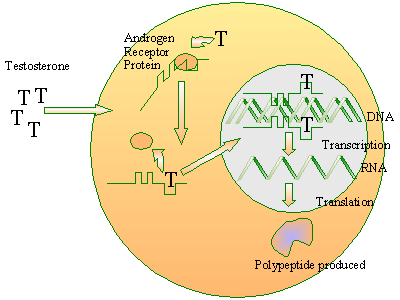 Testosterone exerts its effect in a somewhat indirect fashion. When
testosterone reaches a target organ or target tissue, it must be absorbed
into the cells of that tissue. Inside those cells is a kind of
protein,
coded for by the person’s DNA, called an androgen receptor which, as
its name implies, receives the testosterone and binds on to it. As the
testosterone binds onto the androgen receptor protein, it causes a change in
that protein’s native conformation which converts the inactive receptor into
an active DNA-binding state, thereby enabling the protein to chemically
interact with that cell’s DNA. Thus, once testosterone has attached to the
androgen receptor, that pair goes into the nucleus of the cell and interacts
with the person’s DNA, thereby controlling
transcription
of other genes. Many of those genes control “male” traits such as embryonic
development of male external genitalia.
Testosterone exerts its effect in a somewhat indirect fashion. When
testosterone reaches a target organ or target tissue, it must be absorbed
into the cells of that tissue. Inside those cells is a kind of
protein,
coded for by the person’s DNA, called an androgen receptor which, as
its name implies, receives the testosterone and binds on to it. As the
testosterone binds onto the androgen receptor protein, it causes a change in
that protein’s native conformation which converts the inactive receptor into
an active DNA-binding state, thereby enabling the protein to chemically
interact with that cell’s DNA. Thus, once testosterone has attached to the
androgen receptor, that pair goes into the nucleus of the cell and interacts
with the person’s DNA, thereby controlling
transcription
of other genes. Many of those genes control “male” traits such as embryonic
development of male external genitalia.
All human embryos, whether XX or XY, develop identically for the first 6 weeks
of life, all have undifferentiated external genitalia, and all have
rudimentary primordial gonadal tissue that can, potentially, form either male
or female organs. If the embryo baby has a Y chromosome, that Y chromosome
contains a gene which codes for the formation of testes from the primordial
gonadal tissue at about 6 weeks. Testes, by the way, form in approximately
the same location in the abdomen as ovaries do, with the difference that
while ovaries “stay put,” normally, testes later move down lower in the
abdomen and eventually, out the bottom of the abdomen and into the scrotum
(thus, we say they “descend”). Formation of the testes is not dependent on
androgens such as testosterone, but rather, once testes have begun to form,
they start to secrete androgens, including testosterone, as well as another
hormone called anti-Müllerian hormone. The anti-Müllerian
hormone has an inhibitory effect which causes regression of the
primordial female system, thus inhibiting the development of Fallopian tubes,
uterus, and the upper portion of the vagina. The androgens, including
testosterone, have a stimulatory effect on development of the male
system, which causes development of the epididymis, vasa deferentia, and
seminal vesicles during about the 9th through 13th
weeks. In the absence of the effects of these hormones, development of the
male system does not occur and instead, by “default,” the female external
genitalia (labia, vagina) develop.
However, in order for embryonic development of the male organs to take place,
the androgen receptor protein has to be functioning properly. As just
mentioned, in type II (adult onset) diabetes, the person’s body is
making enough insulin, but the insulin receptors in that person’s liver
cannot properly receive the insulin. Similarly, the androgen receptors must
be functioning properly to receive testosterone. Since the androgen
receptor molecules are a kind of protein, that means they’re under the
control of the gene that codes for them, and any
mutation
of that gene – totally missing androgen receptor gene, missing chunk of gene,
frameshift mutation, etc. – can cause the protein to be absent or have an
abnormal native conformation that is incapable of binding on to testosterone.
Thus, even though lots of testosterone is present, the androgen receptor
can’t bind on to it, and therefore is unable to control transcription of
other genes. This would make that person’s organs/tissues appear to be
totally resistant or insensitive to the effects of testosterone, hence the
name “Androgen Insensitivity Syndrome.”
Now, consider the effect that would have on the embryonic development of an
XY individual. Since the Y chromosome is present, that person has the gene
to make testes, so the testes begin to develop and start to secrete
testosterone and anti-Müllerian hormone. Since the anti-Müllerian
hormone is functioning and received properly, development of the uterus,
Fallopian tubes, and the top end of the vagina will be inhibited. However,
despite lots of testosterone, the rest of the body never gets the message,
so the epididymis, vasa deferentia, and seminal vesicles will not develop.
Also, without the effects of testosterone, external male genitalia (scrotum,
penis) will not form, but rather by “default,” as is normally the case in
the absence of the influence of testosterone, the external genitalia will be
totally female, including the labia and most of the vagina. Thus, even
though this person is chromosomally XY and has testes, she is
phenotypically female. Actually, since a girl/woman with AIS is
totally resistant to the effects of testosterone, it’s kind-of like she’s
more female than a “typical” XX female whose phenotype is influenced by the
testosterone in her body. Usually her testes do not descend, but remain in
her abdomen, and thus, as with any undescended testes, they are more likely
to develop testicular cancer. When this baby is born, to her doctors, nurses,
and parents she looks like any other normal little girl, but her undescended
testes may be discovered later if they are in such a position as to give
the appearance of a hernia.
What about later in life? In some girls with AIS, their undescended testes
are never apparent, and the condition is discovered when they fail to begin
menstruating despite normal body development at puberty. Even XY men
produce some estrogen in their bodies. In the bodies of women with AIS,
some of the testosterone they produce is converted to estrogen, and that,
coupled with the estrogen being produced by their bodies is enough that
their estrogen levels are about the same as an XX woman in the ”follicular“
phase of her
monthly cycle,
and women with AIS go through normal development at puberty (breast development,
widening of the hips, etc.). Actually, since the effects of estrogen are
unopposed by testosterone in their bodies, breast development is often more
significant than XX women whose development is also influenced by
testosterone. Since testosterone plays a role in teenage acne, women with
AIS typically have very clear, acne-free complexions. Since the testes do
produce some estrogen, and since some of the testosterone they produce is
converted to estrogen, their presence in her body can aid in development at
puberty, but due to the increased risk of testicular cancer in undescended
testes, physicians often encourage their removal soon thereafter. Since she
doesn’t have a uterus or ovaries, a woman with AIS will not menstruate and
will not be able to become pregnant, thus may want to consider adopting
children. Since, as mentioned above, growth of axillary and pubic hair is
controlled by testosterone, women with AIS will usually not have that type
of coarse hair, which can be very upsetting to a teen being ridiculed by her
classmates during gym class showers. Depending on the shortness of the
woman’s vagina, once she is sexually active, that may help to stretch it,
but in some cases, a doctor might advise surgery to lengthen it.
While a girl who has AIS has enough estrogen in her body to stimulate normal
(or greater than normal) breast development, size-wise, she lacks the
hormones needed to stimulate development of the actual mammary gland tissue.
If, however, she is given supplemental hormones during puberty, mammary
gland tissue will properly develop, and as an adult, she will be as capable
as any other adoptive mother of nursing a baby.
The genetics of AIS is an intriguing part of this story. As mentioned above,
AIS may be attributed to a mutation in the gene that codes for the androgen
receptor protein. Thus, AIS is, essentially, an allele that influences the
sex of the individual, but interestingly, AIS is also an X-linked,
recessive allele. In other words, testosterone sensitivity is coded for by
a gene on the X chromosome (and the recessive allele codes for a
non-functioning receptor). If we let XA represent the allele that
codes for functional androgen receptor and Xa represent the
allele that codes for non-functional androgen receptor, then a person who is
XAXA would be a female who is normally receptive to
testosterone. Someone who is XAXa would be a
carrier female, and because this is an X- linked gene, her body would
be a mosaic of tissue types – some of her cells would be sensitive to
testosterone while others would be resistant, depending on which X chromosome
was active and which had become a Barr body. Some women who are
heterozygous
have delayed
menarche
(onset of menstruation) or may have reduced or asymmetrical development of
pubic or axillary hair. Someone who is XAY would be a male who
is normally receptive to testosterone. However, unlike other sex-linked
alleles, because AIS affects the sex of the person, someone who is
XaY would be a female with AIS. If a carrier woman and a
man get married, the Punnett square for their children would look like:
| |
XA |
Y |
| XA |
XAXA |
XAY |
| Xa |
XAXa |
XaY |
Thus, ľ of their children would be expected to be girls and only Ľ boys. Of
the girls, we would expect ⅓ to have normal testosterone
receptors, ⅓ to be carriers, and ⅓ to have AIS (of all the
children, that would be Ľ each).
When we discussed other sex-linked genes such as hemophilia, red-green
colorblindness, and white-eyed fruit flies, we took things one step farther,
and showed how a carrier female (XX') and affected male (X'Y) could produce
a homozygous recessive female (X'X') offspring. Because AIS affects the sex
of the individual, that genetic cross wouldn’t be possible. First of all,
someone who is X'Y would be female, not male, and so most likely would get
married to a man (XY), not another woman (XX'). Secondly, since she doesn’t
have a uterus, she can’t get pregnant. Thirdly, between the fact that her
testes, if not surgically removed, are undescended (and therefore sterile)
and the fact that her testes, along with the rest of her body, are
insensitive to the effects of testosterone (and therefore sterile), they
would produce no sperm (and anyway, sperm would have no way out). Unlike
other X-linked alleles, a girl would not inherit this from her father.
An X'X' individual would be extremely rare because no one could inherit that
combination, so the only way to get that would be in the extremely unlikely
event that mutations suddenly occurred in the X chromosomes that both
parents gave to that daughter.
Several famous actresses and female athletes supposedly have AIS. Actually,
the idea of a successful female athlete with AIS is of interest because it is
generally thought that athletic prowess is related to testosterone, yet here
is a woman whose body is totally unaffected by testosterone. Because
athletic organizations, including the Olympics, have not understood that,
these women have, at times, been unfairly barred from competition, based
solely on the fact that they have a Y chromosome, and they have been forced
to bring law suits to be permitted to compete. Because of our society’s
overall lack of understanding and acceptance, some doctors try to convince
parents of an androgen-insensitive daughter to keep that a secret from her,
but that never works. Sooner or later, she will find out or figure it out,
somehow, often accompanied by feelings of guilt, embarassment, and
bewilderment, so wouldn’t it be much better to hear it gradually from loving,
supportive parents as she grows up than to, as a young adult, suddenly hear
it from someone else?
Some things to discuss with your study group: what would you do if...?
- What if one of your neighbors or another student
here at school told you that his/her daughter has AIS? What would you
think? What would go through your mind when you met that girl for the first
time? What would you say to your own children about her?
- Suppose you are the parent of a cute little girl
(pick an age: 2? 4? 8?) who loves dressing up in frilly dresses and playing
with baby dolls and who, you were told, had a hernia that would have to be
surgically corrected. What if the doctor came out of surgery to tell you
your little girl did not have a hernia, but they had just discovered she had
testes? What if the doctor, then, recommended getting a karyotype done, and
the results came back saying that chromosomally, she’s XY? How would you
feel? What would go through your mind?
- What would you say to her as she was growing up?
What would you say to her when she told you she was going to be a mommy and
have babies when she grows up? What would you say to her before or while
she was taking junior high health class? What would you say to her when she
didn’t start menstruating like all her peers, so they were making fun of her?
What would you say to her when she didn’t want to go to junior-high gym class
because, during the mandatory group showers afterwards, the other girls
ridiculed her because she lacked pubic hair? What would you say to her when
she was going through all the insecurities and self-doubts that teenagers go
through?
- One for the men: suppose you started dating a
woman, fell deeply in love with her, and the two of you were thinking about
getting married. What if, in the course of a conversation one day, she told
you that she had AIS, that her karyotype was XY, and that she was scheduled
to have surgery next week to remove her undescended testes? What would you
think? What would you say to her? What would you do?
- One for the women: there is an analogous
condition in which a person can be XX, yet due to influence, or lack thereof,
of the sex hormones, be phenotypically male. I recall being told about a
medical case history where a couple was not having success conceiving a
child, and so they were undergoing medical testing to determine the cause.
In the course of the testing, it was discovered that the man was XX. How do
you think you would react if that was your husband?
References:
Borror, Donald J. 1960. Dictionary of Root Words and Combining Forms. Mayfield Publ. Co.
Brewster, Hugh. 1996. Anastasia’s Album. Madison Press Books, Toronto, Ontario
Campbell, Neil A., Lawrence G. Mitchell, Jane B. Reece. 1999. Biology, 5th Ed. Benjamin/Cummings Publ. Co., Inc. Menlo Park, CA. (plus earlier editions)
Campbell, Neil A., Lawrence G. Mitchell, Jane B. Reece. 1999. Biology: Concepts and Connections, 3rd Ed. Benjamin/Cummings Publ. Co., Inc. Menlo Park, CA. (plus earlier editions)
Marchuk, William N. 1992. A Life Science Lexicon. Wm. C. Brown Publishers, Dubuque, IA.
Quigley, Charmian A., Alessandra De Bellis, Keith B. Marschke, Mostafa K. El-Awady, Elizabeth M. Wilson, and Frank S. French. 1995. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocrine Reviews. 16(3): 271-321.
Copyright © 1996 by J. Stein Carter. All rights reserved.
This page has been accessed  times since 14 Mar 2001.
times since 14 Mar 2001.






